Depletion of γδ T Cells Leads to Reduced Angiogenesis and Increased Infiltration of Inflammatory M1-like Macrophages in Ischemic Muscle Tissue
- PMID: 35563796
- PMCID: PMC9102774
- DOI: 10.3390/cells11091490
Depletion of γδ T Cells Leads to Reduced Angiogenesis and Increased Infiltration of Inflammatory M1-like Macrophages in Ischemic Muscle Tissue
Abstract
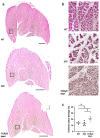
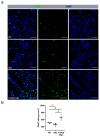
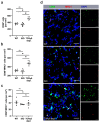
γδ T cells, a small subset of T cells in blood, play a substantial role in influencing immunoregulatory and inflammatory processes. The functional impact of γδ T cells on angiogenesis in ischemic muscle tissue has never been reported and is the topic of the present work. Femoral artery ligation (FAL) was used to induce angiogenesis in the lower leg of γδ T cell depleted mice and wildtype and isotype antibody-treated control groups. Gastrocnemius muscle tissue was harvested 3 and 7 days after FAL and assessed using (immuno-)histological analyses. Hematoxylin and Eosin staining showed an increased area of tissue damage in γδ T cell depleted mice 7 days after FAL. Impaired angiogenesis was demonstrated by lower capillary to muscle fiber ratio and decreased number of proliferating endothelial cells (CD31+/BrdU+). γδ T cell depleted mice showed an increased number of total leukocytes (CD45+), neutrophils (MPO+) and neutrophil extracellular traps (NETs) (MPO+/CitH3+), without changes in the neutrophils to NETs ratio. Moreover, the depletion resulted in a higher macrophage count (DAPI/CD68+) caused by an increase in inflammatory M1-like macrophages (CD68+/MRC1-). Altogether, we show that depletion of γδ T cells leads to increased accumulation of leukocytes and M1-like macrophages, along with impaired angiogenesis.
Keywords: NETs; angiogenesis; gamma delta T cells; ischemia; macrophage polarization; macrophages; neutrophil extracellular traps; neutrophils; proliferation; γδ T cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
C3 Deficiency Leads to Increased Angiogenesis and Elevated Pro-Angiogenic Leukocyte Recruitment in Ischemic Muscle Tissue.Int J Mol Sci. 2021 May 28;22(11):5800. doi: 10.3390/ijms22115800. Int J Mol Sci. 2021. PMID: 34071589 Free PMC article.
-
The Absence of Extracellular Cold-Inducible RNA-Binding Protein (eCIRP) Promotes Pro-Angiogenic Microenvironmental Conditions and Angiogenesis in Muscle Tissue Ischemia.Int J Mol Sci. 2021 Aug 31;22(17):9484. doi: 10.3390/ijms22179484. Int J Mol Sci. 2021. PMID: 34502391 Free PMC article.
-
Absence of Cold-Inducible RNA-Binding Protein (CIRP) Promotes Angiogenesis and Regeneration of Ischemic Tissue by Inducing M2-Like Macrophage Polarization.Biomedicines. 2021 Apr 7;9(4):395. doi: 10.3390/biomedicines9040395. Biomedicines. 2021. PMID: 33916904 Free PMC article.
-
Impact of C57BL/6J and SV-129 Mouse Strain Differences on Ischemia-Induced Postnatal Angiogenesis and the Associated Leukocyte Infiltration in a Murine Hindlimb Model of Ischemia.Int J Mol Sci. 2021 Oct 30;22(21):11795. doi: 10.3390/ijms222111795. Int J Mol Sci. 2021. PMID: 34769229 Free PMC article.
-
RNase A Treatment Interferes With Leukocyte Recruitment, Neutrophil Extracellular Trap Formation, and Angiogenesis in Ischemic Muscle Tissue.Front Physiol. 2020 Nov 6;11:576736. doi: 10.3389/fphys.2020.576736. eCollection 2020. Front Physiol. 2020. PMID: 33240100 Free PMC article.
Cited by
-
Amyotrophic Lateral Sclerosis Pathoetiology and Pathophysiology: Roles of Astrocytes, Gut Microbiome, and Muscle Interactions via the Mitochondrial Melatonergic Pathway, with Disruption by Glyphosate-Based Herbicides.Int J Mol Sci. 2022 Dec 29;24(1):587. doi: 10.3390/ijms24010587. Int J Mol Sci. 2022. PMID: 36614029 Free PMC article. Review.
-
Signal regulatory protein α dynamically mediates macrophage polarization facilitated alleviation of ischemic diseases.Cell Biosci. 2024 Dec 20;14(1):150. doi: 10.1186/s13578-024-01325-2. Cell Biosci. 2024. PMID: 39707436 Free PMC article.
-
State of the Art of Innate Immunity-An Overview.Cells. 2022 Aug 30;11(17):2705. doi: 10.3390/cells11172705. Cells. 2022. PMID: 36078113 Free PMC article.
-
Uncovering a new mechanism of ischemic stroke: a study of the association between γδ T cells and immunoinflammation.Front Immunol. 2025 Jul 17;16:1583274. doi: 10.3389/fimmu.2025.1583274. eCollection 2025. Front Immunol. 2025. PMID: 40746532 Free PMC article. Review.
-
Rag1 Deficiency Impairs Arteriogenesis in Mice.Int J Mol Sci. 2023 Aug 16;24(16):12839. doi: 10.3390/ijms241612839. Int J Mol Sci. 2023. PMID: 37629019 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

