The Role of N6-Methyladenosine in the Promotion of Hepatoblastoma: A Critical Review
- PMID: 35563821
- PMCID: PMC9101889
- DOI: 10.3390/cells11091516
The Role of N6-Methyladenosine in the Promotion of Hepatoblastoma: A Critical Review
Abstract
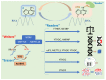
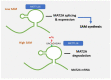
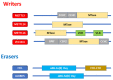
Hepatoblastoma (HB) is a rare primary malignancy of the developing fetal liver. Its course is profoundly influenced by genetics, in the context of sporadic mutation or genetic syndromes. Conventionally, subtypes of HB are histologically determined based on the tissue type that is recapitulated by the tumor and the direction of its differentiation. This classification is being reevaluated based on advances on molecular pathology. The therapeutic approach comprises surgical intervention, chemotherapy (in a neoadjuvant or post-operative capacity), and in some cases, liver transplantation. Although diagnostic modalities and treatment options are evolving, some patients experience complications, including relapse, metastatic spread, and suboptimal response to chemotherapy. As yet, there is no consistent framework with which such outcomes can be predicted. N6-methyladenosine (m6A) is an RNA modification with rampant involvement in the normal processing of cell metabolism and neoplasia. It has been observed to impact the development of a variety of cancers via its governance of gene expression. M6A-associated genes appear prominently in HB. Literature data seem to underscore the role of m6A in promotion and clinical course of HB. Illuminating the pathogenetic mechanisms that drive HB are promising additions to the understanding of the clinically aggressive tumor behavior, given its potential to predict disease course and response to therapy. Implicated genes may also act as targets to facilitate the evolving personalized cancer therapy. Here, we explore the role of m6A and its genetic associates in the promotion of HB, and the impact this may have on the management of this neoplastic disease.
Keywords: N6-Methyladenosine; beta-catenin; hepatoblastoma; hepatoblastoma genetics; m6A; methyltransferase.
Conflict of interest statement
C. M. Sergi receives royalties from Springer and Nova publishers. All royalties go to pediatric charities.
Figures
References
-
- Lachance E., Mandziuk J., Sergi C.M., Bateman J., Low G. Radiologic-Pathologic Correlation of Liver Tumors. In: Sergi C.M., editor. Liver Cancer. Exon Publications; Brisbane, QLD, Australia: 2021. - PubMed
-
- Hager J., Sergi C.M. Hepatoblastoma. In: Sergi C.M., editor. Liver Cancer. Exon Publications; Brisbane, QLD, Australia: 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

