Focused Ultrasound Treatment of a Spheroid In Vitro Tumour Model
- PMID: 35563823
- PMCID: PMC9099905
- DOI: 10.3390/cells11091518
Focused Ultrasound Treatment of a Spheroid In Vitro Tumour Model
Abstract
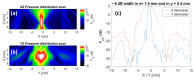
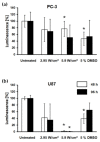
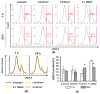
Focused ultrasound (FUS) is a non-invasive technique producing a variety of biological effects by either thermal or mechanical mechanisms of ultrasound interaction with the targeted tissue. FUS could bring benefits, e.g., tumour sensitisation, immune stimulation, and targeted drug delivery, but investigation of FUS effects at the cellular level is still missing. New techniques are commonly tested in vitro on two-dimensional (2D) monolayer cancer cell culture models. The 3D tumour model-spheroid-is mainly utilised to mimic solid tumours from an architectural standpoint. It is a promising method to simulate the characteristics of tumours in vitro and their various responses to therapeutic alternatives. This study aimed to evaluate the effects of FUS on human prostate and glioblastoma cancer tumour spheroids in vitro. The experimental follow-up enclosed the measurements of spheroid integrity and growth kinetics, DNA damage, and cellular metabolic activity by measuring intracellular ATP content in the spheroids. Our results showed that pulsed FUS treatment induced molecular effects in 3D tumour models. With the disruption of the spheroid integrity, we observed an increase in DNA double-strand breaks, leading to damage in the cancer cells depending on the cancer cell type.
Keywords: FUS; focused ultrasound; glioblastoma; in vitro experiments; prostate cancer; spheroid.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

