The Molecular Mechanisms Governing the Assembly of the Immuno- and Thymoproteasomes in the Presence of Constitutive Proteasomes
- PMID: 35563886
- PMCID: PMC9105311
- DOI: 10.3390/cells11091580
The Molecular Mechanisms Governing the Assembly of the Immuno- and Thymoproteasomes in the Presence of Constitutive Proteasomes
Abstract
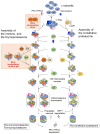
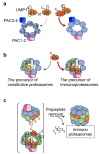
The proteasome is a large protein complex responsible for proteolysis in cells. Though the proteasome is widely conserved in all eukaryotes, vertebrates additionally possess tissue-specific proteasomes, termed immunoproteasomes and thymoproteasomes. These specialized proteasomes diverge from constitutive proteasomes in the makeup of their catalytic 20S core particle (CP), whereby the constitutive β1, β2, and β5 catalytic subunits are replaced by β1i, β2i, and β5i in immunoproteasomes, or β1i, β2i, and β5t in thymoproteasomes. However, as constitutive β1, β2, and β5 are also present in tissues and cells expressing immuno- and thymoproteasomes, the specialized proteasomes must be able to selectively incorporate their specific subunits. Here, we review the mechanisms governing the assembly of constitutive and specialized proteasomes elucidated thus far. Studies have revealed that β1i and β2i are added onto the α-ring of the CP prior to the other β subunits. Furthermore, β5i and β5t can be incorporated independent of β4, whereas constitutive β5 incorporation is dependent on β4. These mechanisms allow the immuno- and thymoproteasomes to integrate tissue-specific β-subunits without contamination from constitutive β1, β2, and β5. We end the review with a brief discussion on the diseases caused by mutations to the immunoproteasome and the proteins involved with its assembly.
Keywords: PAC1–PAC2; PAC3–PAC4; UMP1; chaperone; immunoproteasome; intermediate proteasome; propeptide; proteasome; thymoproteasome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Assembly mechanisms of specialized core particles of the proteasome.Biomolecules. 2014 Jul 16;4(3):662-77. doi: 10.3390/biom4030662. Biomolecules. 2014. PMID: 25033340 Free PMC article.
-
Exclusive expression of proteasome subunit {beta}5t in the human thymic cortex.Blood. 2009 May 21;113(21):5186-91. doi: 10.1182/blood-2008-11-187633. Epub 2009 Mar 16. Blood. 2009. PMID: 19289856
-
Functional Differences between Proteasome Subtypes.Cells. 2022 Jan 26;11(3):421. doi: 10.3390/cells11030421. Cells. 2022. PMID: 35159231 Free PMC article. Review.
-
Efficiency of the four proteasome subtypes to degrade ubiquitinated or oxidized proteins.Sci Rep. 2020 Sep 25;10(1):15765. doi: 10.1038/s41598-020-71550-5. Sci Rep. 2020. PMID: 32978409 Free PMC article.
-
Origin and evolution of the specialized forms of proteasomes involved in antigen presentation.Immunogenetics. 2019 Mar;71(3):251-261. doi: 10.1007/s00251-019-01105-0. Epub 2019 Jan 24. Immunogenetics. 2019. PMID: 30675634 Free PMC article. Review.
Cited by
-
Visualizing chaperone-mediated multistep assembly of the human 20S proteasome.Nat Struct Mol Biol. 2024 Aug;31(8):1176-1188. doi: 10.1038/s41594-024-01268-9. Epub 2024 Apr 10. Nat Struct Mol Biol. 2024. PMID: 38600324 Free PMC article.
-
Mechanism of autocatalytic activation during proteasome assembly.Nat Struct Mol Biol. 2024 Aug;31(8):1167-1175. doi: 10.1038/s41594-024-01262-1. Epub 2024 Apr 10. Nat Struct Mol Biol. 2024. PMID: 38600323 Free PMC article.
-
Molecular insights into the unique properties of the blood-circulating proteasome.J Extracell Biol. 2025 Jan 27;4(1):e70034. doi: 10.1002/jex2.70034. eCollection 2025 Jan. J Extracell Biol. 2025. PMID: 39872464 Free PMC article.
-
Visualizing chaperone-mediated multistep assembly of the human 20S proteasome.bioRxiv [Preprint]. 2024 Jan 28:2024.01.27.577538. doi: 10.1101/2024.01.27.577538. bioRxiv. 2024. Update in: Nat Struct Mol Biol. 2024 Aug;31(8):1176-1188. doi: 10.1038/s41594-024-01268-9. PMID: 38328185 Free PMC article. Updated. Preprint.
-
The Effect of a TLR3 Agonist on Airway Allergic Inflammation and Viral Infection in Immunoproteasome-Deficient Mice.Viruses. 2024 Aug 29;16(9):1384. doi: 10.3390/v16091384. Viruses. 2024. PMID: 39339860 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

