Exploring the Influence of Synthesis Parameters on the Optical Properties for Various CeO2 NPs
- PMID: 35564111
- PMCID: PMC9100359
- DOI: 10.3390/nano12091402
Exploring the Influence of Synthesis Parameters on the Optical Properties for Various CeO2 NPs
Abstract
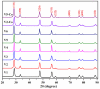
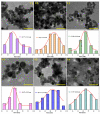
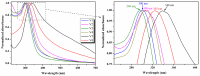
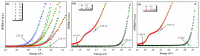
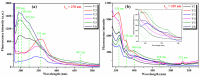
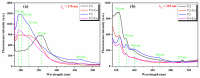
Cerium oxide (CeO2) nanoparticles were synthesized with a chemical precipitation method in different experimental conditions using cerium nitrate hexahydrate (Ce(NO3)3·6H2O) as a precursor, modifying the solution pH, the reaction time, and Co atoms as dopants, in order to tune the band gap energy values of the prepared samples. The physical characteristics of the synthesized ceria nanoparticles were evaluated by Fourier transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), UV-Vis analyses and photoluminescence measurements. XRD data revealed a pure cubic fluorite structure of CeO2 NPs, the estimation of crystallite sizes by Scherrer's formula indicates the formation of crystals with dimensions between 11.24 and 21.65 nm. All samples contain nearly spherical CeO2 nanoparticles, as well as cubic, rhomboidal, triangular, or polyhedral nanoparticles that can be identified by TEM images. The optical investigation of CeO2 samples revealed that the band gap energy values are between 3.18 eV and 2.85 eV, and, after doping with Co atoms, the Eg of samples decreased to about 2.0 eV. In this study, we managed to obtain CeO2 NPs with Eg under 3.0 eV by only modifying the synthesis parameters. In addition, by doping with Co ions, the band gap energy value was lowered to 2.0 eV. This aspect leads to promising results that provide an encouraging approach for future photocatalytic investigations.
Keywords: CeO2 nanoparticles; Co-doping; band gap tuning; optical properties; pH variation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Ultra-fast photocatalytic degradation and seed germination of band gap tunable nickel doping ceria nanoparticles.Chemosphere. 2023 Aug;333:138934. doi: 10.1016/j.chemosphere.2023.138934. Epub 2023 May 12. Chemosphere. 2023. PMID: 37182707
-
Nano-catalytic behavior of CeO2 nanoparticles in dye adsorption: Synthesis through bio-combustion and assessment of UV-light-driven photo-adsorption of indigo carmine dye.Heliyon. 2024 Jul 31;10(15):e35505. doi: 10.1016/j.heliyon.2024.e35505. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39165952 Free PMC article.
-
Bio-therapeutic Potential and Cytotoxicity Assessment of Pectin-Mediated Synthesized Nanostructured Cerium Oxide.Appl Biochem Biotechnol. 2016 Oct;180(4):638-654. doi: 10.1007/s12010-016-2121-9. Epub 2016 May 27. Appl Biochem Biotechnol. 2016. PMID: 27234032
-
[Study on the preparation and spectral characteristics of CeO2 nanocrystallines].Guang Pu Xue Yu Guang Pu Fen Xi. 2009 Nov;29(11):3011-4. Guang Pu Xue Yu Guang Pu Fen Xi. 2009. PMID: 20101975 Chinese.
-
The Effect of Precursor Concentration on the Particle Size, Crystal Size, and Optical Energy Gap of CexSn1-xO2 Nanofabrication.Nanomaterials (Basel). 2021 Aug 22;11(8):2143. doi: 10.3390/nano11082143. Nanomaterials (Basel). 2021. PMID: 34443973 Free PMC article.
References
-
- Amoresi R.A.C., Oliveira R.C., Marana N.L., De Almeida P.B., Prata P.S., Zaghete M.A., Longo E., Sambrano J.R., Simões A.Z. CeO2 nanoparticle morphologies and their corresponding crystalline planes for the photocatalytic degradation of organic pollutants. ACS Appl. Nano Mater. 2019;2:6513–6526. doi: 10.1021/acsanm.9b01452. - DOI
-
- Trovarelli A., Llorca J. Ceria catalysts at nanoscale: How do crystal shapes shape catalysis? ACS Catal. 2017;7:4716–4735. doi: 10.1021/acscatal.7b01246. - DOI
-
- Mullins D.R., Albrecht P.M., Calaza F. Variations in reactivity on different crystallographic orientations of cerium oxide. Top. Catal. 2013;56:1345–1362. doi: 10.1007/s11244-013-0146-7. - DOI
-
- Ma Y., Gao W., Zhang Z., Zhang S., Tian Z., Liu Y., Ho J.C., Qu Y. Regulating the surface of nanoceria and its applications in heterogeneous catalysis. Surf. Sci. Rep. 2018;73:1–36. doi: 10.1016/j.surfrep.2018.02.001. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

