Impact of Adsorption of Straight Chain Alcohol Molecules on the Optical Properties of Calcite (10.4) Surface
- PMID: 35564169
- PMCID: PMC9099925
- DOI: 10.3390/nano12091460
Impact of Adsorption of Straight Chain Alcohol Molecules on the Optical Properties of Calcite (10.4) Surface
Abstract
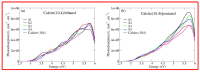
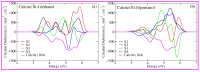
Calcium carbonate plays a central role in controlling the chemistry of the oceans, biomineralization and oil production, to name a few. In this work, using density functional theory with semiempirical dispersion corrections and simplified TD-DFT using Tamm-Dancoff approximation, we investigated the impact of the adsorption of straight chain alcohol (ethanol and pentanol) molecules on the optical properties of a calcite (10.4) surface. Our results show that ethanol and/or pentanol molecules form a well-ordered monolayer (through their hydroxyl group with carbon chains sticking away in a standing-up position) on the calcite (10.4) surface. Additionally, we found intriguing modulations in the photoabsorption spectra and circular dichroism spectra. In particular, the latter was a unique optical fingerprint for a molecule-adsorbed calcite (10.4) surface. Our findings provide useful insights into the structural and optical features of calcite-based systems at the atomic level.
Keywords: DFT; TD-DFT; adsorption; calcite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Unravelling the impact of oily alkane molecules on the optical properties of the calcite(10.4) surface.Phys Chem Chem Phys. 2023 May 3;25(17):12192-12199. doi: 10.1039/d3cp00626c. Phys Chem Chem Phys. 2023. PMID: 37073534
-
Interaction of alcohols with the calcite surface.Phys Chem Chem Phys. 2015 Feb 7;17(5):3490-6. doi: 10.1039/c4cp05235h. Epub 2014 Dec 23. Phys Chem Chem Phys. 2015. PMID: 25533590
-
Binding of ethanol on calcite: the role of the OH bond and its relevance to biomineralization.Langmuir. 2010 Oct 5;26(19):15239-47. doi: 10.1021/la101136j. Langmuir. 2010. PMID: 20812690
-
Growth and Dissolution of Calcite in the Presence of Adsorbed Stearic Acid.Langmuir. 2015 Jul 14;31(27):7563-71. doi: 10.1021/acs.langmuir.5b01732. Epub 2015 Jun 30. Langmuir. 2015. PMID: 26087312
-
Probing the energetics of organic-nanoparticle interactions of ethanol on calcite.Proc Natl Acad Sci U S A. 2015 Apr 28;112(17):5314-8. doi: 10.1073/pnas.1505874112. Epub 2015 Apr 13. Proc Natl Acad Sci U S A. 2015. PMID: 25870281 Free PMC article.
References
-
- Millero F.J. Chemical Oceanography. 4th ed. Elsevier; Amsterdam, The Netherlands: 2013.
-
- Morse J.W., Mackenzie F.T. Geochemistry of Sedimentary Carbonates. Elsevier; Amsterdam, The Netherlands: 1990.
-
- Talham D.R. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry Stephen Mann. Oxford University Press, New York, USA, 2001. Cryst. Growth Des. 2002;2:675. doi: 10.1021/cg020033l. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

