Microwave-Assisted Synthesis of Reduced Graphene Oxide with Hollow Nanostructure for Application to Lithium-Ion Batteries
- PMID: 35564216
- PMCID: PMC9103021
- DOI: 10.3390/nano12091507
Microwave-Assisted Synthesis of Reduced Graphene Oxide with Hollow Nanostructure for Application to Lithium-Ion Batteries
Abstract
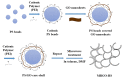
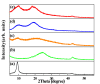
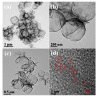
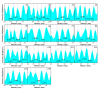
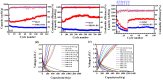
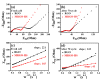
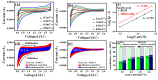
In this study, reduced graphene oxide (RGO) with a hollow nanostructure was successfully synthesized by layer-by-layer self-assembly using electrostatic interactions and van der Waals forces between building blocks, and its lithium storage characteristics were investigated. After 800 cycles at a current density of 1 A/g, the microwave-irradiated RGO hollow spheres (MRGO-HS) maintained a capacity of 626 mA h/g. In addition, when the charge/discharge capacity was measured stepwise in the current density range of 0.1-2 A/g, the discharge capacity of the RGO rapidly decreased to 156 mA h/g even at the current density of 2 A/g, whereas MRGO-HS provided a capacity of 252 mA h/g. Even after the current density was restored at a current density of 0.1 A/g, the MRGO-HS capacity was maintained to be 827 mA h/g at the 100th cycle, which is close to the original reversible capacity. Thus, MRGO-HS provides a higher capacity and better rate capability than those of traditionally synthesized RGO.
Keywords: Exfoliation; hollow spheres; layer-by-layer self-assembly; lithium-ion batteries; reduced graphene oxide.
Conflict of interest statement
The authors declare no conflict of interest. And the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Hollow Nanobarrels of α-Fe2O3 on Reduced Graphene Oxide as High-Performance Anode for Lithium-Ion Batteries.ACS Appl Mater Interfaces. 2016 Jan 27;8(3):2027-34. doi: 10.1021/acsami.5b10342. Epub 2016 Jan 12. ACS Appl Mater Interfaces. 2016. PMID: 26717009
-
CuCo2O4 Hollow Microspheres with Graphene Composite Targeting Superior Lithium-Ion Storage.Langmuir. 2021 Jul 20;37(28):8426-8434. doi: 10.1021/acs.langmuir.1c00670. Epub 2021 Jul 7. Langmuir. 2021. PMID: 34233119
-
Facile synthesis and electrochemical performances of hollow graphene spheres as anode material for lithium-ion batteries.Nanoscale Res Lett. 2014 Jul 28;9(1):368. doi: 10.1186/1556-276X-9-368. eCollection 2014. Nanoscale Res Lett. 2014. PMID: 25114657 Free PMC article.
-
Size-controllable synthesis of Zn2GeO4 hollow rods supported on reduced graphene oxide as high-capacity anode for lithium-ion batteries.J Colloid Interface Sci. 2021 May;589:13-24. doi: 10.1016/j.jcis.2020.12.121. Epub 2021 Jan 2. J Colloid Interface Sci. 2021. PMID: 33450456
-
Multiphase and Double-Layer NiFe2O4@NiO-Hollow-Nanosphere-Decorated Reduced Graphene Oxide Composite Powders Prepared by Spray Pyrolysis Applying Nanoscale Kirkendall Diffusion.ACS Appl Mater Interfaces. 2015 Aug 5;7(30):16842-9. doi: 10.1021/acsami.5b04891. Epub 2015 Jul 24. ACS Appl Mater Interfaces. 2015. PMID: 26186601
Cited by
-
Dynamics of reduced graphene oxide: synthesis and structural models.RSC Adv. 2023 Jun 12;13(26):17633-17655. doi: 10.1039/d3ra02098c. eCollection 2023 Jun 9. RSC Adv. 2023. PMID: 37312999 Free PMC article. Review.
-
The Efficiency Study of Graphene Synthesis on Copper Substrate via Chemical Vapor Deposition Method with Methanol Precursor.Nanomaterials (Basel). 2023 Mar 22;13(6):1136. doi: 10.3390/nano13061136. Nanomaterials (Basel). 2023. PMID: 36986030 Free PMC article.
-
Electrodeposition and Properties of Composite Ni Coatings Modified with Multilayer Graphene Oxide.Micromachines (Basel). 2023 Sep 7;14(9):1747. doi: 10.3390/mi14091747. Micromachines (Basel). 2023. PMID: 37763910 Free PMC article.
References
-
- Peng K., Jie J., Zhang W., Lee S.-T. Silicon nanowires for rechargeable lithium-ion battery anodes. Appl. Phys. Lett. 2008;93:033105. doi: 10.1063/1.2929373. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

