Intracellular Trafficking and Distribution of Cd and InP Quantum Dots in HeLa and ML-1 Thyroid Cancer Cells
- PMID: 35564224
- PMCID: PMC9104504
- DOI: 10.3390/nano12091517
Intracellular Trafficking and Distribution of Cd and InP Quantum Dots in HeLa and ML-1 Thyroid Cancer Cells
Abstract
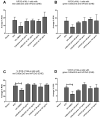
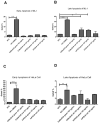
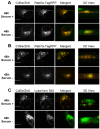
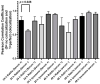
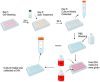
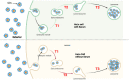
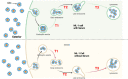
The study of the interaction of engineered nanoparticles, including quantum dots (QDs), with cellular constituents and the kinetics of their localization and transport, has provided new insights into their biological consequences in cancers and for the development of effective cancer therapies. The present study aims to elucidate the toxicity and intracellular transport kinetics of CdSe/ZnS and InP/ZnS QDs in late-stage ML-1 thyroid cancer using well-tested HeLa as a control. Our XTT (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) viability assay (Cell Proliferation Kit II) showed that ML-1 cells and non-cancerous mouse fibroblast cells exhibit no viability defect in response to these QDs, whereas HeLa cell viability decreases. These results suggest that HeLa cells are more sensitive to the QDs compared to ML-1 cells. To test the possibility that transporting rates of QDs are different between HeLa and ML-1 cells, we performed a QD subcellular localization assay by determining Pearson's Coefficient values and found that HeLa cells showed faster QDs transporting towards the lysosome. Consistently, the ICP-OES test showed the uptake of CdSe/ZnS QDs in HeLa cells was significantly higher than in ML-1 cells. Together, we conclude that high levels of toxicity in HeLa are positively correlated with the traffic rate of QDs in the treated cells.
Keywords: CdSe/ZnS; HeLa; InP/ZnS; ML-1; cancer; distribution; quantum dots; trafficking.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures









Similar articles
-
A Comparison of Common Quantum Dot Alternatives to Cadmium-Based Quantum Dots on the Basis of Liver Cytotoxicity.Nanomaterials (Basel). 2024 Jun 25;14(13):1086. doi: 10.3390/nano14131086. Nanomaterials (Basel). 2024. PMID: 38998690 Free PMC article.
-
Cytotoxicity assessment of functionalized CdSe, CdTe and InP quantum dots in two human cancer cell models.Mater Sci Eng C Mater Biol Appl. 2015 Dec 1;57:222-31. doi: 10.1016/j.msec.2015.07.044. Epub 2015 Jul 26. Mater Sci Eng C Mater Biol Appl. 2015. PMID: 26354258
-
Cellular uptake, elimination and toxicity of CdSe/ZnS quantum dots in HepG2 cells.Biomaterials. 2013 Dec;34(37):9545-58. doi: 10.1016/j.biomaterials.2013.08.038. Epub 2013 Sep 5. Biomaterials. 2013. PMID: 24011712
-
Exploring quantitative cellular bioimaging and assessment of CdSe/ZnS quantum dots cellular uptake in single cells, using ns-LA-ICP-SFMS.Talanta. 2021 May 15;227:122162. doi: 10.1016/j.talanta.2021.122162. Epub 2021 Feb 9. Talanta. 2021. PMID: 33714466
-
Microenvironmental Impact on InP/ZnS-Based Quantum Dots in In Vitro Models and in Living Cells: Spectrally- and Time-Resolved Luminescence Analysis.Int J Mol Sci. 2023 Jan 31;24(3):2699. doi: 10.3390/ijms24032699. Int J Mol Sci. 2023. PMID: 36769021 Free PMC article.
Cited by
-
The Impact of Cadmium Selenide Zinc Sulfide Quantum Dots on the Proteomic Profile of Saccharomyces cerevisiae.Int J Mol Sci. 2023 Nov 15;24(22):16332. doi: 10.3390/ijms242216332. Int J Mol Sci. 2023. PMID: 38003523 Free PMC article.
-
A Review of in vivo Toxicity of Quantum Dots in Animal Models.Int J Nanomedicine. 2023 Dec 29;18:8143-8168. doi: 10.2147/IJN.S434842. eCollection 2023. Int J Nanomedicine. 2023. PMID: 38170122 Free PMC article. Review.
-
CdSe/ZnS Quantum Dots' Impact on In Vitro Actin Dynamics.Int J Mol Sci. 2024 Apr 10;25(8):4179. doi: 10.3390/ijms25084179. Int J Mol Sci. 2024. PMID: 38673765 Free PMC article.
-
A Comparison of Common Quantum Dot Alternatives to Cadmium-Based Quantum Dots on the Basis of Liver Cytotoxicity.Nanomaterials (Basel). 2024 Jun 25;14(13):1086. doi: 10.3390/nano14131086. Nanomaterials (Basel). 2024. PMID: 38998690 Free PMC article.
-
Exploring the potential and safety of quantum dots in allergy diagnostics.Microsyst Nanoeng. 2023 Nov 17;9:145. doi: 10.1038/s41378-023-00608-x. eCollection 2023. Microsyst Nanoeng. 2023. PMID: 38025887 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

