Evaluation of Celiac Disease by Minimally Invasive Biomarkers in a Spanish Pediatric Population
- PMID: 35564413
- PMCID: PMC9100138
- DOI: 10.3390/ijerph19095020
Evaluation of Celiac Disease by Minimally Invasive Biomarkers in a Spanish Pediatric Population
Abstract
Background: The diagnosis of celiac disease (CD) has been substantially improved with the availability of highly sensitive CD-specific IgA-TG2, Ig-GDP, and IgA-EMA. The European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) published (2012) and updated (2020) diagnostic criteria for CD in order to simplify CD diagnosis and to avoid biopsies in selected patients.
Methods: A prospective study including 5641 pediatric patients (0-16 years old) from January 2012 to January 2019 was performed. CD diagnosis was made according to the ESPGHAN algorithm. The objective of this study was to evaluate the utility of biomarkers and the relationship between TGA-IgA and EMA titers.
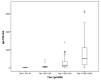
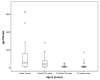
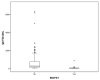
Results: CD diagnoses were confirmed in 113 patients, 110 were IgA-TG2-positive and 3 (2.7%) had IgA deficiency. The diagnosis was made by serologic tests in 95 (84.1%) patients. Only 18 (15.9%) patients underwent intestinal biopsy. We obtained 100% concordance between IgA-EMA and positive results for IgA-TG2 ≥ 10 ULN with IgA-EMA antibody titer ≥ 1:80.
Conclusions: This study provides evidence of a positive correlation between IgA-TG2 antibody serum levels and IgA-EMA. The diagnosis could be guaranteed with strict application of IgA-TG2 values ≥ 10 ULN (confirmed by subsequent testing) plus the serological response to the gluten-free diet (GFD).
Keywords: ESPGHAN; antibodies; celiac disease; diagnosis; non-invasive biomarkers; pediatric age.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Accuracy in Diagnosis of Celiac Disease Without Biopsies in Clinical Practice.Gastroenterology. 2017 Oct;153(4):924-935. doi: 10.1053/j.gastro.2017.06.002. Epub 2017 Jun 15. Gastroenterology. 2017. PMID: 28624578
-
Evaluation of the ESPGHAN Celiac Guidelines in a North American Pediatric Population.Am J Gastroenterol. 2015 May;110(5):760-7. doi: 10.1038/ajg.2015.87. Epub 2015 Mar 31. Am J Gastroenterol. 2015. PMID: 25823767
-
AGA Clinical Practice Update on Diagnosis and Monitoring of Celiac Disease-Changing Utility of Serology and Histologic Measures: Expert Review.Gastroenterology. 2019 Mar;156(4):885-889. doi: 10.1053/j.gastro.2018.12.010. Epub 2018 Dec 19. Gastroenterology. 2019. PMID: 30578783 Free PMC article. Review.
-
Outcome in pediatric celiac disease is independent of the diagnostic approach in patients with high antibody levels.J Pediatr Gastroenterol Nutr. 2024 Jul;79(1):84-91. doi: 10.1002/jpn3.12251. Epub 2024 May 20. J Pediatr Gastroenterol Nutr. 2024. PMID: 38769762
-
Tests for Serum Transglutaminase and Endomysial Antibodies Do Not Detect Most Patients With Celiac Disease and Persistent Villous Atrophy on Gluten-free Diets: a Meta-analysis.Gastroenterology. 2017 Sep;153(3):689-701.e1. doi: 10.1053/j.gastro.2017.05.015. Epub 2017 May 22. Gastroenterology. 2017. PMID: 28545781 Free PMC article. Review.
References
-
- Werkstetter K.J., Korponay-Szabó I.R., Popp A., Villanacci V., Salemme M., Heilig G., Lillevang S.T., Mearin M.L., Ribes-Koninckx C., Thomas A., et al. Accuracy in diagnosis of celiac disease without biopsies in clinical practice. Gastroenterology. 2017;153:924–935. doi: 10.1053/j.gastro.2017.06.002. - DOI - PubMed
-
- Saginur M., Alrefaee F.A., Spady D.W., Girgis S.A., Huynh H.Q., Prosser C.I., Persad R., Turner J.M. Antitissue transglutaminase antibody determination versus upper endoscopic bi-opsy diagnosis of paediatric celiac disease. Paediatr. Child Health. 2013;18:246–250. doi: 10.1093/pch/18.5.246. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

