Effects of Pyrolysis Temperature and Chemical Modification on the Adsorption of Cd and As(V) by Biochar Derived from Pteris vittata
- PMID: 35564620
- PMCID: PMC9104657
- DOI: 10.3390/ijerph19095226
Effects of Pyrolysis Temperature and Chemical Modification on the Adsorption of Cd and As(V) by Biochar Derived from Pteris vittata
Abstract
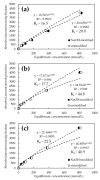
Phytoremediation can be applied successfully to solve the serious worldwide issue of arsenic (As) and cadmium (Cd) pollution. However, the treatment of biomass containing toxic elements after remediation is a challenge. In this study, we investigated the effective use of biomass resources by converting the As hyperaccumulator P. vittata into biochar to adsorb toxic elements. Plant biomass containing As was calcined at 600, 800, and 1200 °C, and its surface structure and adsorption performances for As(V) and Cd were evaluated. Pyrolysis at 1200 °C increased the specific surface area of the biochar, but it did not significantly affect its adsorption capacity for toxic elements. The calcined biochar had very high adsorption capacities of 90% and 95% for As(V) and Cd, respectively, adsorbing 6000 mmol/g-biochar for As(V) and 4000 mmol/g-biochar for Cd. The As(V) adsorption rate was improved by FeCl3 treatment. However, the adsorption capacity for Cd was not significantly affected by the NaOH treatment. In conclusion, it was found that after phytoremediation using P. vittata biomass, it can be effectively used as an environmental purification material by conversion to biochar. Furthermore, chemical modification with FeCl3 improves the biochar's adsorption performance.
Keywords: arsenic; biochar; cadmium; phytoremediation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Modeling the removal of Cd(II) and Pb(II) from aqueous solutions by biochar derived from arsenic hyperaccumulator Pteris vittata.J Environ Manage. 2025 May;381:125280. doi: 10.1016/j.jenvman.2025.125280. Epub 2025 Apr 9. J Environ Manage. 2025. PMID: 40199226
-
Potential in treating arsenic-contaminated water of the biochars produced from hyperaccumulator Pteris vittata and its environmental safety.Environ Pollut. 2024 Sep 1;356:124320. doi: 10.1016/j.envpol.2024.124320. Epub 2024 Jun 4. Environ Pollut. 2024. PMID: 38844037
-
Adsorption antagonism and synergy of arsenate(V) and cadmium(II) onto Fe-modified rice straw biochars.Environ Geochem Health. 2019 Aug;41(4):1755-1766. doi: 10.1007/s10653-017-9984-8. Epub 2017 May 26. Environ Geochem Health. 2019. PMID: 28550600
-
[Modified Biochar for Remediation of Soil Contaminated with Arsenic and Cadmium: A Review].Huan Jing Ke Xue. 2023 Jul 8;44(7):4077-4090. doi: 10.13227/j.hjkx.202207032. Huan Jing Ke Xue. 2023. PMID: 37438305 Review. Chinese.
-
A critical review of the arsenic uptake mechanisms and phytoremediation potential of Pteris vittata.Int J Phytoremediation. 2014;16(5):429-53. doi: 10.1080/15226514.2013.798613. Int J Phytoremediation. 2014. PMID: 24912227 Review.
Cited by
-
The Mechanism of Arsenic Release in Contaminated Paddy Soil with Added Biochar: The Role of Dissolved Organic Matter, Fe, and Bacteria.Toxics. 2024 Sep 10;12(9):661. doi: 10.3390/toxics12090661. Toxics. 2024. PMID: 39330589 Free PMC article.
References
-
- Hans Wedepohl K. The composition of the continental crust. Geochim. Cosmochim. Acta. 1995;59:1217–1232. doi: 10.1016/0016-7037(95)00038-2. - DOI
-
- Argos M., Kalra T., Rathouz P.J., Chen Y., Pierce B., Parvez F., Islam T., Ahmed A., Rakibuz-Zaman M., Hasan R., et al. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): A prospective cohort study. Lancet. 2010;376:252–258. doi: 10.1016/S0140-6736(10)60481-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

