Vaccinomics to Design a Multi-Epitopes Vaccine for Acinetobacter baumannii
- PMID: 35564967
- PMCID: PMC9104312
- DOI: 10.3390/ijerph19095568
Vaccinomics to Design a Multi-Epitopes Vaccine for Acinetobacter baumannii
Abstract
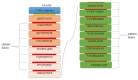
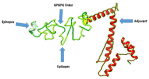
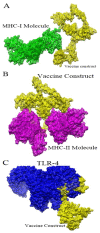
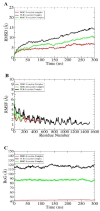
Antibiotic resistance (AR) is the result of microbes' natural evolution to withstand the action of antibiotics used against them. AR is rising to a high level across the globe, and novel resistant strains are emerging and spreading very fast. Acinetobacter baumannii is a multidrug resistant Gram-negative bacteria, responsible for causing severe nosocomial infections that are treated with several broad spectrum antibiotics: carbapenems, β-lactam, aminoglycosides, tetracycline, gentamicin, impanel, piperacillin, and amikacin. The A. baumannii genome is superplastic to acquire new resistant mechanisms and, as there is no vaccine in the development process for this pathogen, the situation is more worrisome. This study was conducted to identify protective antigens from the core genome of the pathogen. Genomic data of fully sequenced strains of A. baumannii were retrieved from the national center for biotechnological information (NCBI) database and subjected to various genomics, immunoinformatics, proteomics, and biophysical analyses to identify potential vaccine antigens against A. baumannii. By doing so, four outer membrane proteins were prioritized: TonB-dependent siderphore receptor, OmpA family protein, type IV pilus biogenesis stability protein, and OprD family outer membrane porin. Immuoinformatics predicted B-cell and T-cell epitopes from all four proteins. The antigenic epitopes were linked to design a multi-epitopes vaccine construct using GPGPG linkers and adjuvant cholera toxin B subunit to boost the immune responses. A 3D model of the vaccine construct was built, loop refined, and considered for extensive error examination. Disulfide engineering was performed for the stability of the vaccine construct. Blind docking of the vaccine was conducted with host MHC-I, MHC-II, and toll-like receptors 4 (TLR-4) molecules. Molecular dynamic simulation was carried out to understand the vaccine-receptors dynamics and binding stability, as well as to evaluate the presentation of epitopes to the host immune system. Binding energies estimation was achieved to understand intermolecular interaction energies and validate docking and simulation studies. The results suggested that the designed vaccine construct has high potential to induce protective host immune responses and can be a good vaccine candidate for experimental in vivo and in vitro studies.
Keywords: Acinetobacter baumannii; core genomics; epitope vaccine; molecular dynamics simulations; pan-genomics.
Conflict of interest statement
The author declares no conflict of interest.
Figures

References
-
- PCAST . National Action Plan for Combatting Antibiotic-Resistant Bacteria. White House; Washington, DC, USA: 2015.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

