Systemic Steroids in Preventing Bronchopulmonary Dysplasia (BPD): Neurodevelopmental Outcome According to the Risk of BPD in the EPICE Cohort
- PMID: 35564997
- PMCID: PMC9106050
- DOI: 10.3390/ijerph19095600
Systemic Steroids in Preventing Bronchopulmonary Dysplasia (BPD): Neurodevelopmental Outcome According to the Risk of BPD in the EPICE Cohort
Abstract
Background: Postnatal steroids (PNS) have been used to prevent bronchopulmonary dysplasia (BPD) in preterm infants but have potential adverse effects on neurodevelopment. These effects might be modulated by their risk of BPD. We aimed to compare patients' neurodevelopment with PNS treatment according to their risk of BPD in a European cohort.
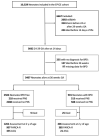
Methods: We developed a prediction model for BPD to classify infants born between 24 + 0 and 29 + 6 weeks of gestation in three groups and compared patients' neurological outcome at two years of corrected age using the propensity score (PS) method.
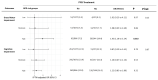
Results: Of 3662 neonates included in the analysis, 901 (24.6%) were diagnosed with BPD. Our prediction model for BPD had an area under the ROC curve of 0.82. In the group with the highest risk of developing BPD, PNS were associated with an increased risk of gross motor impairment: OR of 1.95 after IPTW adjustment (95% CI 1.18 to 3.24, p = 0.010). This difference existed regardless of the type of steroid used. However, there was an increased risk of cognitive anomalies for patients treated with dexa/betamethasone that was no longer observed with hydrocortisone.
Conclusions: This study suggests that PNS might be associated with an increased risk of gross motor impairment regardless of the group risk for BPD. Further randomised controlled trials exploring the use of PNS to prevent BPD should include a risk-based evaluation of neurodevelopmental outcomes. This observation still needs to be confirmed in a randomised controlled trial.
Keywords: bronchopulmonary dysplasia; neurodevelopmental outcome; post natal steroid therapy; preterm birth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Short E.J., Kirchner H.L., Asaad G.R., Fulton S.E., Lewis B.A., Klein N., Eisengart S., Baley J., Kercsmar C., Min M.O., et al. Developmental sequelae in preterm infants having a diagnosis of bronchopulmonary dysplasia: Analysis using a severity-based classification system. Arch. Pediatr. Adolesc. Med. 2007;161:1082–1087. doi: 10.1001/archpedi.161.11.1082. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

