The Effect of Hyperthermia and Radiotherapy Sequence on Cancer Cell Death and the Immune Phenotype of Breast Cancer Cells
- PMID: 35565180
- PMCID: PMC9103710
- DOI: 10.3390/cancers14092050
The Effect of Hyperthermia and Radiotherapy Sequence on Cancer Cell Death and the Immune Phenotype of Breast Cancer Cells
Abstract
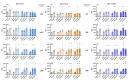
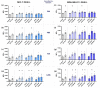
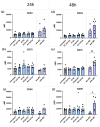
Hyperthermia (HT) is an accepted treatment for recurrent breast cancer which locally heats the tumor to 39-44 °C, and it is a very potent sensitizer for radiotherapy (RT) and chemotherapy. However, currently little is known about how HT with a distinct temperature, and particularly, how the sequence of HT and RT changes the immune phenotype of breast cancer cells. Therefore, human MDA-MB-231 and MCF-7 breast cancer cells were treated with HT of different temperatures (39, 41 and 44 °C), alone and in combination with RT (2 × 5 Gy) in different sequences, with either RT or HT first, followed by the other. Tumor cell death forms and the expression of immune checkpoint molecules (ICMs) were analyzed by multicolor flow cytometry. Human monocyte-derived dendritic cells (moDCs) were differentiated and co-cultured with the treated cancer cells. In both cell lines, RT was the main stressor for cell death induction, with apoptosis being the prominent cell death form in MCF-7 cells and both apoptosis and necrosis in MDA-MB-231 cells. Here, the sequence of the combined treatments, either RT or HT, did not have a significant impact on the final outcome. The expression of all of the three examined immune suppressive ICMs, namely PD-L1, PD-L2 and HVEM, was significantly increased on MCF-7 cells 120 h after the treatment of RT with HT of any temperature. Of special interest for MDA-MB-231 cells is that only combinations of RT with HT of both 41 and 44 °C induced a significantly increased expression of PD-L2 at all examined time points (24, 48, 72, and 120 h). Generally, high dynamics of ICM expression can be observed after combined RT and HT treatments. There was no significant difference between the different sequences of treatments (either HT + RT or RT + HT) in case of the upregulation of ICMs. Furthermore, the co-culture of moDCs with tumor cells of any treatment had no impact on the expression of activation markers. We conclude that the sequence of HT and RT does not strongly affect the immune phenotype of breast cancer cells. However, when HT is combined with RT, it results in an increased expression of distinct immune suppressive ICMs that should be considered by including immune checkpoint inhibitors in multimodal tumor treatments with RT and HT. Further, combined RT and HT affects the immune system in the effector phase rather than in the priming phase.
Keywords: breast cancer; dendritic cell activation; hyperthermia; hyperthermia treatment sequence; immune checkpoint molecules; immune phenotype; radiotherapy.
Conflict of interest statement
The authors declare no conflict of interest with regard to the work presented here.
Figures



Similar articles
-
Detailed in vitro analyses of the impact of multimodal cancer therapy with hyperthermia and radiotherapy on the immune phenotype of human glioblastoma cells.Int J Hyperthermia. 2022;39(1):796-805. doi: 10.1080/02656736.2022.2080873. Int J Hyperthermia. 2022. PMID: 35676615
-
Clonogenicity-based radioresistance determines the expression of immune suppressive immune checkpoint molecules after hypofractionated irradiation of MDA-MB-231 triple-negative breast cancer cells.Front Oncol. 2023 Apr 20;13:981239. doi: 10.3389/fonc.2023.981239. eCollection 2023. Front Oncol. 2023. PMID: 37152024 Free PMC article.
-
Differences of the Immune Phenotype of Breast Cancer Cells after Ex Vivo Hyperthermia by Warm-Water or Microwave Radiation in a Closed-Loop System Alone or in Combination with Radiotherapy.Cancers (Basel). 2020 Apr 27;12(5):1082. doi: 10.3390/cancers12051082. Cancers (Basel). 2020. PMID: 32349284 Free PMC article.
-
Temperature and thermal dose during radiotherapy and hyperthermia for recurrent breast cancer are related to clinical outcome and thermal toxicity: a systematic review.Int J Hyperthermia. 2019;36(1):1024-1039. doi: 10.1080/02656736.2019.1665718. Int J Hyperthermia. 2019. PMID: 31621437
-
Biological rationales and clinical applications of temperature controlled hyperthermia--implications for multimodal cancer treatments.Curr Med Chem. 2010;17(27):3045-57. doi: 10.2174/092986710791959774. Curr Med Chem. 2010. PMID: 20629627 Review.
Cited by
-
Hydrogel systems for targeted cancer therapy.Front Bioeng Biotechnol. 2023 Feb 16;11:1140436. doi: 10.3389/fbioe.2023.1140436. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 36873346 Free PMC article. Review.
-
Oncologic Thermoradiotherapy: Need for Evidence, Harmonisation, and Innovation.Cancers (Basel). 2022 May 13;14(10):2418. doi: 10.3390/cancers14102418. Cancers (Basel). 2022. PMID: 35626026 Free PMC article.
-
The potential of gas plasma technology for targeting breast cancer.Clin Transl Med. 2022 Aug;12(8):e1022. doi: 10.1002/ctm2.1022. Clin Transl Med. 2022. PMID: 35994412 Free PMC article. Review.
-
The Rationale for Combining Hypofractionated Radiation and Hyperthermia.Cancers (Basel). 2024 Nov 22;16(23):3916. doi: 10.3390/cancers16233916. Cancers (Basel). 2024. PMID: 39682105 Free PMC article. Review.
-
Radiotherapy combined with locoregional hyperthermia for oligoprogression in metastatic melanoma local control.Ther Adv Med Oncol. 2025 Feb 12;17:17588359251316189. doi: 10.1177/17588359251316189. eCollection 2025. Ther Adv Med Oncol. 2025. PMID: 39943943 Free PMC article.
References
-
- Villacampa G., Tolosa P., Salvador F., Sánchez-Bayona R., Villanueva L., Dienstmann R., Ciruelos E., Pascual T. Addition of immune checkpoint inhibitors to chemotherapy versus chemotherapy alone in first-line metastatic triple-negative breast cancer: A systematic review and meta-analysis. Cancer Treat. Rev. 2022;104:102352. doi: 10.1016/j.ctrv.2022.102352. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

