The Bone Marrow Microenvironment in B-Cell Development and Malignancy
- PMID: 35565219
- PMCID: PMC9102980
- DOI: 10.3390/cancers14092089
The Bone Marrow Microenvironment in B-Cell Development and Malignancy
Abstract
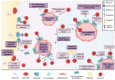
B lymphopoiesis is characterized by progressive loss of multipotent potential in hematopoietic stem cells, followed by commitment to differentiate into B cells, which mediate the humoral response of the adaptive immune system. This process is tightly regulated by spatially distinct bone marrow niches where cells, including mesenchymal stem and progenitor cells, endothelial cells, osteoblasts, osteoclasts, and adipocytes, interact with B-cell progenitors to direct their proliferation and differentiation. Recently, the B-cell niche has been implicated in initiating and facilitating B-cell precursor acute lymphoblastic leukemia. Leukemic cells are also capable of remodeling the B-cell niche to promote their growth and survival and evade treatment. Here, we discuss the major cellular components of bone marrow niches for B lymphopoiesis and the role of the malignant B-cell niche in disease development, treatment resistance and relapse. Further understanding of the crosstalk between leukemic cells and bone marrow niche cells will enable development of additional therapeutic strategies that target the niches in order to hinder leukemia progression.
Keywords: B-cell acute lymphoblastic leukemia (B-ALL); B-cell development; B-cell niche; bone marrow (BM); bone marrow microenvironment (BMM); leukemia.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Patient-Derived Bone Marrow Spheroids Reveal Leukemia-Initiating Cells Supported by Mesenchymal Hypoxic Niches in Pediatric B-ALL.Front Immunol. 2021 Oct 19;12:746492. doi: 10.3389/fimmu.2021.746492. eCollection 2021. Front Immunol. 2021. PMID: 34737747 Free PMC article.
-
Remodeling of the bone marrow microenvironment during acute myeloid leukemia progression.Ann Transl Med. 2024 Aug 1;12(4):63. doi: 10.21037/atm-23-1824. Epub 2024 Jan 15. Ann Transl Med. 2024. PMID: 39118939 Free PMC article. Review.
-
The Bone Marrow Niche in B-Cell Acute Lymphoblastic Leukemia: The Role of Microenvironment from Pre-Leukemia to Overt Leukemia.Int J Mol Sci. 2021 Apr 23;22(9):4426. doi: 10.3390/ijms22094426. Int J Mol Sci. 2021. PMID: 33922612 Free PMC article. Review.
-
Toward Therapeutic Targeting of Bone Marrow Leukemic Niche Protective Signals in B-Cell Acute Lymphoblastic Leukemia.Front Oncol. 2021 Jan 8;10:606540. doi: 10.3389/fonc.2020.606540. eCollection 2020. Front Oncol. 2021. PMID: 33489914 Free PMC article. Review.
-
Advances in understanding the acute lymphoblastic leukemia bone marrow microenvironment: From biology to therapeutic targeting.Biochim Biophys Acta. 2016 Mar;1863(3):449-463. doi: 10.1016/j.bbamcr.2015.08.015. Epub 2015 Sep 1. Biochim Biophys Acta. 2016. PMID: 26334291 Review.
Cited by
-
A comprehensive prognostic and immune analysis of LAPTM4B in pan-cancer and Philadelphia chromosome-positive acute lymphoblastic leukemia.Front Immunol. 2025 Feb 28;16:1522293. doi: 10.3389/fimmu.2025.1522293. eCollection 2025. Front Immunol. 2025. PMID: 40092987 Free PMC article.
-
Favorable and poor prognosis B-cell precursor acute lymphoblastic leukemia subtypes reveal distinct leukemic cell properties when interacting with mesenchymal stem cells, differentially modifying their cell stemness and leukemia chemoresistance.J Cell Commun Signal. 2025 Jun 12;19(2):e70009. doi: 10.1002/ccs3.70009. eCollection 2025 Jun. J Cell Commun Signal. 2025. PMID: 40511387 Free PMC article.
-
Murine bone-derived mesenchymal stem cells undergo molecular changes after a single passage in culture.Sci Rep. 2024 May 29;14(1):12396. doi: 10.1038/s41598-024-63009-8. Sci Rep. 2024. PMID: 38811646 Free PMC article.
-
Targeting IL-17A enhances imatinib efficacy in Philadelphia chromosome-positive B-cell acute lymphoblastic leukemia.Nat Commun. 2024 Jan 3;15(1):203. doi: 10.1038/s41467-023-44270-3. Nat Commun. 2024. PMID: 38172124 Free PMC article.
-
Immunogenic shift of arginine metabolism triggers systemic metabolic and immunological reprogramming to prevent HER2+ breast cancer.bioRxiv [Preprint]. 2024 Oct 26:2024.10.23.619827. doi: 10.1101/2024.10.23.619827. bioRxiv. 2024. Update in: Cancer Metab. 2025 Mar 20;13(1):15. doi: 10.1186/s40170-025-00384-4. PMID: 39484369 Free PMC article. Updated. Preprint.
References
-
- Adolfsson J., Mansson R., Buza-Vidas N., Hultquist A., Liuba K., Jensen C.T., Bryder D., Yang L., Borge O.-J., Thoren L.A., et al. Identification of Flt3+ Lympho-Myeloid Stem Cells Lacking Erythro-Megakaryocytic Potential: A Revised Road Map for Adult Blood Lineage Commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. - DOI - PubMed
-
- Inlay M.A., Bhattacharya D., Sahoo D., Serwold T., Seita J., Karsunky H., Plevritis S.K., Dill D.L., Weissman I.L. Ly6d marks the earliest stage of B-cell specification and identifies the branchpoint between B-cell and T-cell development. Genes Dev. 2009;23:2376–2381. doi: 10.1101/gad.1836009. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

