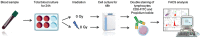Improving Patients' Life Quality after Radiotherapy Treatment by Predicting Late Toxicities
- PMID: 35565227
- PMCID: PMC9099838
- DOI: 10.3390/cancers14092097
Improving Patients' Life Quality after Radiotherapy Treatment by Predicting Late Toxicities
Abstract
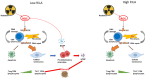
Personalized treatment and precision medicine have become the new standard of care in oncology and radiotherapy. Because treatment outcomes have considerably improved over the last few years, permanent side-effects are becoming an increasingly significant issue for cancer survivors. Five to ten percent of patients will develop severe late toxicity after radiotherapy. Identifying these patients before treatment start would allow for treatment adaptation to minimize definitive side effects that could impair their long-term quality of life. Over the last decades, several tests and biomarkers have been developed to identify these patients. However, out of these, only the Radiation-Induced Lymphocyte Apoptosis (RILA) assay has been prospectively validated in multi-center cohorts. This test, based on a simple blood draught, has been shown to be correlated with late radiation-induced toxicity in breast, prostate, cervical and head and neck cancer. It could therefore greatly improve decision making in precision radiation oncology. This literature review summarizes the development and bases of this assay, as well as its clinical results and compares its results to the other available assays.
Keywords: biomarkers; late toxicities prediction; personalized treatment; radiotherapy.
Conflict of interest statement
D. Azria declares NovaGray, stock options. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures
Similar articles
-
A Review of Radiation-Induced Lymphocyte Apoptosis as a Predictor of Late Toxicity After Breast Radiotherapy.J Med Imaging Radiat Sci. 2019 Jun;50(2):337-344. doi: 10.1016/j.jmir.2019.02.004. Epub 2019 Apr 15. J Med Imaging Radiat Sci. 2019. PMID: 31176443
-
Towards Personalized Radiotherapy in Pelvic Cancer: Patient-Related Risk Factors for Late Radiation Toxicity.Curr Oncol. 2025 Jan 17;32(1):47. doi: 10.3390/curroncol32010047. Curr Oncol. 2025. PMID: 39851963 Free PMC article. Review.
-
Multi-centre technical evaluation of the radiation-induced lymphocyte apoptosis assay as a predictive test for radiotherapy toxicity.Clin Transl Radiat Oncol. 2019 Jun 10;18:1-8. doi: 10.1016/j.ctro.2019.06.001. eCollection 2019 Sep. Clin Transl Radiat Oncol. 2019. PMID: 31341970 Free PMC article.
-
Radiation-induced CD8 T-lymphocyte Apoptosis as a Predictor of Breast Fibrosis After Radiotherapy: Results of the Prospective Multicenter French Trial.EBioMedicine. 2015 Oct 25;2(12):1965-73. doi: 10.1016/j.ebiom.2015.10.024. eCollection 2015 Dec. EBioMedicine. 2015. PMID: 26844275 Free PMC article. Clinical Trial.
-
Cell Senescence-Related Pathways Are Enriched in Breast Cancer Patients With Late Toxicity After Radiotherapy and Low Radiation-Induced Lymphocyte Apoptosis.Front Oncol. 2022 May 24;12:825703. doi: 10.3389/fonc.2022.825703. eCollection 2022. Front Oncol. 2022. PMID: 35686103 Free PMC article.
Cited by
-
Hype or hope? A review of challenges in balancing tumor control and treatment toxicity in breast cancer from the perspective of the radiation oncologist.Clin Transl Oncol. 2024 Mar;26(3):561-573. doi: 10.1007/s12094-023-03287-2. Epub 2023 Jul 28. Clin Transl Oncol. 2024. PMID: 37505372 Review.
-
A simulated annealing-based Bayesian network structure optimization framework for late morbidity prediction with a large prospective dataset.Med Phys. 2025 Jun;52(6):5051-5063. doi: 10.1002/mp.17881. Epub 2025 May 21. Med Phys. 2025. PMID: 40400111 Free PMC article.
-
Radiation Induced Skin Fibrosis (RISF): Opportunity for Angiotensin II-Dependent Intervention.Int J Mol Sci. 2024 Jul 29;25(15):8261. doi: 10.3390/ijms25158261. Int J Mol Sci. 2024. PMID: 39125831 Free PMC article. Review.
-
Dosimetric comparison and prognostic analysis of helical tomotherapy vs. intensity-modulated radiation therapy in locally advanced cervical cancer.Oncol Lett. 2025 Jun 12;30(2):396. doi: 10.3892/ol.2025.15142. eCollection 2025 Aug. Oncol Lett. 2025. PMID: 40552253 Free PMC article.
-
Assessment of the predictive power the radiation-induced lymphocyte apoptosis method in prostate cancer patients.Sci Rep. 2025 Jan 9;15(1):1516. doi: 10.1038/s41598-024-81450-7. Sci Rep. 2025. PMID: 39789068 Free PMC article.
References
-
- Bentzen S.M., Constine L.S., Deasy J., Eisbruch A., Jackson A., Marks L.B., Haken R.T., Yorke E.D. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): An Introduction to the Scientific Issues. Int. J. Radiat. Oncol. 2010;76:S3–S9. doi: 10.1016/j.ijrobp.2009.09.040. - DOI - PMC - PubMed
-
- Baumann M. Impact of Endogenous and Exogenous Factors on Radiation Sequelae. In: Dunst J., Sauer R., editors. Late Sequelae in Oncology. Springer; Berlin/Heidelberg, Germany: 1995. pp. 3–12. - DOI
-
- Holthusen H. Erfahrungen über die Verträglichkeitsgrenze für Röntgenstrahlen und deren Nutzanwendung zur Verhütung von Schäden. Strahlentherapie. 1936;57:254–269.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources