The Mechanisms of lncRNA-Mediated Multidrug Resistance and the Clinical Application Prospects of lncRNAs in Breast Cancer
- PMID: 35565231
- PMCID: PMC9103444
- DOI: 10.3390/cancers14092101
The Mechanisms of lncRNA-Mediated Multidrug Resistance and the Clinical Application Prospects of lncRNAs in Breast Cancer
Erratum in
-
Correction: Ye et al. The Mechanisms of lncRNA-Mediated Multidrug Resistance and the Clinical Application Prospects of lncRNAs in Breast Cancer. Cancers 2022, 14, 2101.Cancers (Basel). 2025 Jun 27;17(13):2179. doi: 10.3390/cancers17132179. Cancers (Basel). 2025. PMID: 40647567 Free PMC article.
Abstract
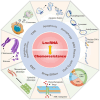
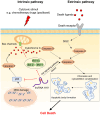
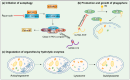
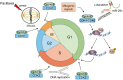
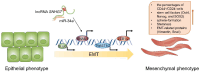
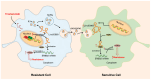
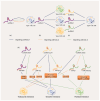
Breast cancer (BC) is a highly heterogeneous disease and presents a great threat to female health worldwide. Chemotherapy is one of the predominant strategies for the treatment of BC; however, multidrug resistance (MDR) has seriously affected or hindered the effect of chemotherapy. Recently, a growing number of studies have indicated that lncRNAs play vital and varied roles in BC chemoresistance, including apoptosis, autophagy, DNA repair, cell cycle, drug efflux, epithelial-mesenchymal transition (EMT), epigenetic modification and the tumor microenvironment (TME). Although thousands of lncRNAs have been implicated in the chemoresistance of BC, a systematic review of their regulatory mechanisms remains to be performed. In this review, we systematically summarized the mechanisms of MDR and the functions of lncRNAs mediated in the chemoresistance of BC from the latest literature. These findings significantly enhance the current understanding of lncRNAs and suggest that they may be promising prognostic biomarkers for BC patients receiving chemotherapy, as well as therapeutic targets to prevent or reverse chemoresistance.
Keywords: MDR; breast cancer; chemoresistance; chemotherapy; exosome; lncRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

