Comparative Effectiveness of Bevacizumab versus Cetuximab in Metastatic Colorectal Cancer Patients without Primary Tumor Resection
- PMID: 35565247
- PMCID: PMC9104998
- DOI: 10.3390/cancers14092118
Comparative Effectiveness of Bevacizumab versus Cetuximab in Metastatic Colorectal Cancer Patients without Primary Tumor Resection
Abstract
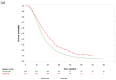
Primary tumor resection may be unfeasible in metastatic colorectal cancer. We determined the effects of bevacizumab and cetuximab therapies on survival or conversion surgery in patients with metastatic colorectal cancer who did not undergo primary tumor resection. This retrospective cohort study enrolled 8466 patients who underwent first-line bevacizumab- or cetuximab-based therapy. We analyzed the data of both therapies in patients who did not undergo primary tumor resection. Overall survival after targeted therapy plus chemotherapy was assessed. The groups were matched using propensity score matching and weighting. Cetuximab resulted in lower mortality than bevacizumab (hazard ratio (HR) = 0.75); however, it did not have the same effect in patients that underwent primary tumor resection (HR = 0.95) after propensity score weighting. Among patients treated with targeted agents, primary tumor resection was associated with lower mortality among those who received both bevacizumab (HR = 0.60) and cetuximab (HR = 0.75). Among patients that did not undergo primary tumor resection, multivariable analysis for conversion surgery showed that the cetuximab group (HR = 1.82) had a significantly higher metastasectomy rate. In these patients, cetuximab-based therapy was associated with significantly better survival compared with bevacizumab-based therapy. Cetuximab also yielded a higher conversion surgery rate. These findings demonstrate the importance of stratification by primary tumor resection in the application of current treatment guidelines and initiation of future clinical trials.
Keywords: bevacizumab; cetuximab; metastatic colorectal cancer; primary tumor resection.
Conflict of interest statement
The funding source was involved in the collection and management of the data. The funding source had no role in the design and conduct of the study; analysis and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. The authors declare no conflict of interest.
Figures


Similar articles
-
Association of Primary Tumor Site With Mortality in Patients Receiving Bevacizumab and Cetuximab for Metastatic Colorectal Cancer.JAMA Surg. 2018 Jan 1;153(1):60-67. doi: 10.1001/jamasurg.2017.3466. JAMA Surg. 2018. PMID: 28975237 Free PMC article.
-
Comparative Effectiveness of Cetuximab Versus Panitumumab in Patients With Metastatic Colorectal Cancer: A Nationwide Database Study.Anticancer Res. 2023 Nov;43(11):5127-5138. doi: 10.21873/anticanres.16713. Anticancer Res. 2023. PMID: 37909992
-
Primary tumor location and survival in colorectal cancer: A retrospective cohort study.World J Gastrointest Oncol. 2020 Apr 15;12(4):405-423. doi: 10.4251/wjgo.v12.i4.405. World J Gastrointest Oncol. 2020. PMID: 32368319 Free PMC article.
-
Integration of novel agents in the treatment of colorectal cancer.Cancer Chemother Pharmacol. 2004 Sep;54 Suppl 1:S32-9. doi: 10.1007/s00280-004-0884-0. Cancer Chemother Pharmacol. 2004. PMID: 15309512 Review.
-
First-line cetuximab versus bevacizumab for RAS and BRAF wild-type metastatic colorectal cancer: a systematic review and meta-analysis.BMC Cancer. 2019 Mar 28;19(1):280. doi: 10.1186/s12885-019-5481-z. BMC Cancer. 2019. PMID: 30922269 Free PMC article.
Cited by
-
Survival benefits of para-aortic lymphadenectomy in colorectal cancer with clinically suspected para-aortic lymph node metastasis: a meta-analysis and systematic review.World J Surg Oncol. 2023 Jan 31;21(1):28. doi: 10.1186/s12957-023-02908-y. World J Surg Oncol. 2023. PMID: 36721235 Free PMC article.
-
Clinical and cost-effectiveness analysis of mFOLOFX6 with or without a targeted drug among patients with metastatic colorectal cancer: inverse probability of treatment weighting.Am J Cancer Res. 2023 Sep 15;13(9):4039-4056. eCollection 2023. Am J Cancer Res. 2023. PMID: 37818063 Free PMC article.
-
Clinical Outcomes of Upfront Primary Tumor Resection in Synchronous Unresectable Metastatic Colorectal Cancer.Cancers (Basel). 2023 Oct 19;15(20):5057. doi: 10.3390/cancers15205057. Cancers (Basel). 2023. PMID: 37894424 Free PMC article.
-
Cost-effectiveness of FOLFOX6+Bevacizumab Versus FOLFOX6+Cetuximab in Stage IV Colorectal Cancer Patients in Shiraz, Iran.Cancer Control. 2023 Jan-Dec;30:10732748231180679. doi: 10.1177/10732748231180679. Cancer Control. 2023. PMID: 37314727 Free PMC article.
References
-
- Botrel T.E.A., Clark L.G.O., Paladini L., Clark O.A.C. Efficacy and Safety of Bevacizumab plus Chemotherapy Compared to Chemotherapy Alone in Previously Untreated Advanced or Metastatic Colorectal Cancer: A Systematic Review and Meta-Analysis. BMC Cancer. 2016;16:677. doi: 10.1186/s12885-016-2734-y. - DOI - PMC - PubMed
-
- Wu C.C., Wang J.H., Lin P.C., Liang C.A., Huang C.Y., Lien H.C., Chen C.Y., Chou K.J., Su Y.C. Tumor Sidedness and Efficacy of First-Line Therapy in Patients with RAS/BRAF Wild-Type Metastatic Colorectal Cancer: A Network Meta-Analysis. Crit. Rev. Oncol. Hematol. 2020;145:102823. doi: 10.1016/j.critrevonc.2019.102823. - DOI - PubMed
-
- Kemeny N. Management of Liver Metastases from Colorectal Cancer. Oncology. 2006;20:1161–1176. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

