IGF2BP1 Promotes Proliferation of Neuroendocrine Neoplasms by Post-Transcriptional Enhancement of EZH2
- PMID: 35565249
- PMCID: PMC9131133
- DOI: 10.3390/cancers14092121
IGF2BP1 Promotes Proliferation of Neuroendocrine Neoplasms by Post-Transcriptional Enhancement of EZH2
Abstract
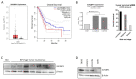
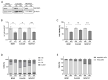
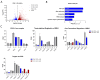
Neuroendocrine neoplasms (NENs) represent a heterogenous class of highly vascularized neoplasms that are increasing in prevalence and are predominantly diagnosed at a metastatic state. The molecular mechanisms leading to tumor initiation, metastasis, and chemoresistance are still under investigation. Hence, identification of novel therapeutic targets is of great interest. Here, we demonstrate that the RNA-binding Protein IGF2BP1 is a post-transcriptional regulator of components of the Polycomb repressive complex 2 (PRC2), an epigenic modifier affecting transcriptional regulation and proliferation: Comprehensive in silico analyses along with in vitro experiments showed that IGF2BP1 promotes neuroendocrine tumor cell proliferation by stabilizing the mRNA of Enhancer of Zeste 2 (EZH2), the catalytic subunit of PRC2, which represses gene expression by tri-methylation of histone H3 at lysine 27 (H3K27me3). The IGF2BP1-driven stabilization and protection of EZH2 mRNA is m6A-dependent and enhances EZH2 protein levels which stimulates cell cycle progression by silencing cell cycle arrest genes through enhanced H3K27 tri-methylation. Therapeutic inhibition of IGF2BP1 destabilizes EZH2 mRNA and results in a reduced cell proliferation, paralleled by an increase in G1 and sub-G1 phases. Combined targeting of IGF2BP1, EZH2, and Myc, a transcriptional activator of EZH2 and well-known target of IGF2BP1 cooperatively induces tumor cell apoptosis. Our data identify IGF2BP1 as an important driver of tumor progression in NEN, and indicate that disruption of the IGF2BP1-Myc-EZH2 axis represents a promising approach for targeted therapy of neuroendocrine neoplasms.
Keywords: EZH2; H3K27me3; IGF2BP1; NEN; RNA-binding protein; cell cycle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bosman F.T., Carneiro F., Hruban R.H., Theise N.D. WHO Classification of Tumours of the Digestive System. 4th ed. Volume 3. International Agency for Research on Cancer; Lyon, France: 2010. pp. 1–417.
LinkOut - more resources
Full Text Sources

