Treatment-Related Adverse Events of Combination EGFR Tyrosine Kinase Inhibitor and Immune Checkpoint Inhibitor in EGFR-Mutant Advanced Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis
- PMID: 35565285
- PMCID: PMC9102470
- DOI: 10.3390/cancers14092157
Treatment-Related Adverse Events of Combination EGFR Tyrosine Kinase Inhibitor and Immune Checkpoint Inhibitor in EGFR-Mutant Advanced Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis
Abstract
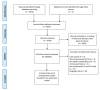
(1) Background: We performed a meta-analysis to examine whether combined epidermal growth factor tyrosine kinase inhibitor (EGFR-TKI) and immune checkpoint inhibitor (ICI) increases treatment-related adverse events (trAEs) in advanced non-small cell lung cancer (NSCLC). (2) Methods: Articles from MEDLINE, EMBASE, and Cochrane databases were searched. Proportions and odds ratios (ORs) of the pooled incidence of overall and organ-specific trAEs in combination EGFR-TKI and ICI were compared to TKI monotherapy. (3) Results: Eight studies fulfilled our selection criteria. Any-grade organ-specific trAEs were more common in combination EGFR-TKI and ICI than TKI monotherapy (skin: OR = 1.19, p = 0.012; gastrointestinal tract: OR = 1.04, p = 0.790; ILD: OR = 1.28, p = 0.001). Grade ≥ 3 trAEs were also more frequent in combination treatment (skin: OR = 1.13, p = 0.082; gastrointestinal tract: OR = 1.13, p = 0.076; ILD: OR = 1.16, p = 0.003). (4) Conclusions: A higher proportion of grade ≥3 skin and gastrointestinal trAEs and ILDs was observed in combination TKI and ICI compared to TKI alone. Caution has to be taken when interpreting the results owing to the small number of studies included in this meta-analysis.
Keywords: EGFR mutation; NSCLC; immune checkpoint inhibitors; meta-analysis; tyrosine kinase inhibitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Shi Y., Au J.S.-K., Thongprasert S., Srinivasan S., Tsai C.-M., Khoa M.T., Heeroma K., Itoh Y., Cornelio G., Yang P.-C. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER) J. Thorac. Oncol. 2014;9:154–162. doi: 10.1097/JTO.0000000000000033. - DOI - PMC - PubMed
-
- Gahr S., Stoehr R., Geissinger E., Ficker J., Brueckl W., Gschwendtner A., Gattenloehner S., Fuchs F., Schulz C., Rieker R. EGFR mutational status in a large series of Caucasian European NSCLC patients: Data from daily practice. Br. J. Cancer. 2013;109:1821–1828. doi: 10.1038/bjc.2013.511. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous