Malignant Superficial Mesenchymal Tumors in Children
- PMID: 35565289
- PMCID: PMC9104419
- DOI: 10.3390/cancers14092160
Malignant Superficial Mesenchymal Tumors in Children
Abstract
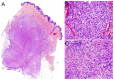
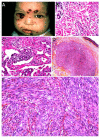
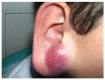
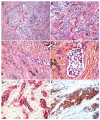
Malignant superficial mesenchymal tumors are a very diverse group of neoplasms with few clinical and radiological discriminatory factors. Hence, some of these cancers are rarely suspected based on clinical and radiological grounds, others may be easily misdiagnosed, and the histological analysis of a biopsy or resection is central in the diagnostic process. In children, the age at presentation is a major element of the differential diagnosis. Some tumors have a very distinct epidemiology, while others may be seen at any age. More recently, the advances in molecular biology have greatly improved the diagnosis of mesenchymal tumors and new entities are still being described. In the present review, we provide an overview of the diversity of malignant superficial mesenchymal tumors in children, including new and/or rare entities. We discuss the important diagnostic features, be they clinical, histological, or molecular. Special attention was given to the genetic features of these tumors, particularly when they were helpful for the diagnosis or treatment.
Keywords: children; diagnosis; genetics; histology; mesenchymal tumors; sarcoma; skin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

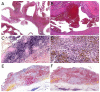


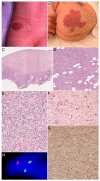
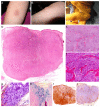
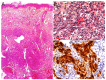


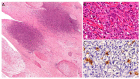
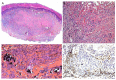

Similar articles
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
Cited by
-
Infantile fibrosarcoma of the left upper limb mimicking a hemangioma: A case report.Int J Surg Case Rep. 2025 Jun;131:111417. doi: 10.1016/j.ijscr.2025.111417. Epub 2025 May 9. Int J Surg Case Rep. 2025. PMID: 40373513 Free PMC article.
References
-
- Amadeo B., Penel N., Coindre J.-M., Ray-Coquard I., Ligier K., Delafosse P., Bouvier A.-M., Plouvier S., Gallet J., Lacourt A., et al. Incidence and Time Trends of Sarcoma (2000–2013): Results from the French Network of Cancer Registries (FRANCIM) BMC Cancer. 2020;20:190. doi: 10.1186/s12885-020-6683-0. - DOI - PMC - PubMed
-
- Saito K., Kobayashi E., Yoshida A., Araki Y., Kubota D., Tanzawa Y., Kawai A., Yanagawa T., Takagishi K., Chuman H. Angiomatoid Fibrous Histiocytoma: A Series of Seven Cases Including Genetically Confirmed Aggressive Cases and a Literature Review. BMC Musculoskelet. Disord. 2017;18:31. doi: 10.1186/s12891-017-1390-y. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

