Targeting Sphingolipid Metabolism as a Therapeutic Strategy in Cancer Treatment
- PMID: 35565311
- PMCID: PMC9104917
- DOI: 10.3390/cancers14092183
Targeting Sphingolipid Metabolism as a Therapeutic Strategy in Cancer Treatment
Abstract
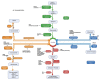
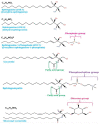
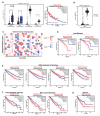
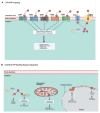
Sphingolipids are bioactive molecules that have key roles in regulating tumor cell death and survival through, in part, the functional roles of ceramide accumulation and sphingosine-1-phosphate (S1P) production, respectively. Mechanistic studies using cell lines, mouse models, or human tumors have revealed crucial roles of sphingolipid metabolic signaling in regulating tumor progression in response to anticancer therapy. Specifically, studies to understand ceramide and S1P production pathways with their downstream targets have provided novel therapeutic strategies for cancer treatment. In this review, we present recent evidence of the critical roles of sphingolipids and their metabolic enzymes in regulating tumor progression via mechanisms involving cell death or survival. The roles of S1P in enabling tumor growth/metastasis and conferring cancer resistance to existing therapeutics are also highlighted. Additionally, using the publicly available transcriptomic database, we assess the prognostic values of key sphingolipid enzymes on the overall survival of patients with different malignancies and present studies that highlight their clinical implications for anticancer treatment.
Keywords: apoptosis; cancer; cell growth; ceramide; sphingolipids; sphingosine-1-phosphate (S1P); therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Sphingolipid metabolism in cancer signalling and therapy.Nat Rev Cancer. 2018 Jan;18(1):33-50. doi: 10.1038/nrc.2017.96. Epub 2017 Nov 17. Nat Rev Cancer. 2018. PMID: 29147025 Free PMC article. Review.
-
Dysregulation of sphingolipid metabolic enzymes leads to high levels of sphingosine-1-phosphate and ceramide in human hepatocellular carcinoma.Hepatol Res. 2021 May;51(5):614-626. doi: 10.1111/hepr.13625. Epub 2021 Mar 9. Hepatol Res. 2021. PMID: 33586816
-
Novel agents targeting bioactive sphingolipids for the treatment of cancer.Curr Med Chem. 2013;20(1):108-22. Curr Med Chem. 2013. PMID: 23244584 Review.
-
Role of sphingosine kinase localization in sphingolipid signaling.World J Biol Chem. 2010 Dec 26;1(12):362-8. doi: 10.4331/wjbc.v1.i12.362. World J Biol Chem. 2010. PMID: 21537471 Free PMC article.
-
Sphingosine kinase/sphingosine 1-phosphate signaling in cancer therapeutics and drug resistance.Handb Exp Pharmacol. 2013;(216):3-27. doi: 10.1007/978-3-7091-1511-4_1. Handb Exp Pharmacol. 2013. PMID: 23563649 Review.
Cited by
-
GBA Regulates EMT/MET and Chemoresistance in Squamous Cell Carcinoma Cells by Modulating the Cellular Glycosphingolipid Profile.Cells. 2023 Jul 18;12(14):1886. doi: 10.3390/cells12141886. Cells. 2023. PMID: 37508550 Free PMC article.
-
Drug-Induced Reorganisation of Lipid Metabolism Limits the Therapeutic Efficacy of Ponatinib in Glioma Stem Cells.Pharmaceutics. 2024 May 29;16(6):728. doi: 10.3390/pharmaceutics16060728. Pharmaceutics. 2024. PMID: 38931850 Free PMC article.
-
The ceramide synthase (CERS/LASS) family: Functions involved in cancer progression.Cell Oncol (Dordr). 2023 Aug;46(4):825-845. doi: 10.1007/s13402-023-00798-6. Epub 2023 Mar 22. Cell Oncol (Dordr). 2023. PMID: 36947340 Review.
-
Sphingolipid Signaling and Complement Activation in Glioblastoma: A Promising Avenue for Therapeutic Intervention.Biochem (Basel). 2024 Jun;4(2):126-143. doi: 10.3390/biochem4020007. Epub 2024 Jun 6. Biochem (Basel). 2024. PMID: 38894892 Free PMC article.
-
Metabolomic profiling of upper GI malignancies in blood and tissue: a systematic review and meta-analysis.J Cancer Res Clin Oncol. 2024 Jul 1;150(7):331. doi: 10.1007/s00432-024-05857-5. J Cancer Res Clin Oncol. 2024. PMID: 38951269 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

