Transcriptomic Profiling of Gene Expression Associated with Granulosa Cell Tumor Development in a Mouse Model
- PMID: 35565312
- PMCID: PMC9105549
- DOI: 10.3390/cancers14092184
Transcriptomic Profiling of Gene Expression Associated with Granulosa Cell Tumor Development in a Mouse Model
Abstract
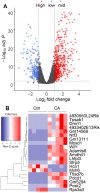
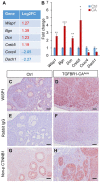
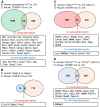
Ovarian granulosa cell tumors (GCTs) are rare sex cord-stromal tumors, accounting for ~5% ovarian tumors. The etiology of GCTs remains poorly defined. Genetically engineered mouse models are potentially valuable for understanding the pathogenesis of GCTs. Mice harboring constitutively active TGFβ signaling (TGFBR1-CA) develop ovarian GCTs that phenocopy several hormonal and molecular characteristics of human GCTs. To determine molecular alterations in the ovary upon TGFβ signaling activation, we performed transcriptomic profiling of gene expression associated with GCT development using ovaries from 1-month-old TGFBR1-CA mice and age-matched controls. RNA-sequencing and bioinformatics analysis coupled with the validation of select target genes revealed dysregulations of multiple cellular events and signaling molecules/pathways. The differentially expressed genes are enriched not only for known GCT-related pathways and tumorigenic events but also for signaling events potentially mediated by neuroactive ligand-receptor interaction, relaxin signaling, insulin signaling, and complements in TGFBR1-CA ovaries. Additionally, a comparative analysis of our data in mice with genes dysregulated in human GCTs or granulosa cells overexpressing a mutant FOXL2, the genetic hallmark of adult GCTs, identified some common genes altered in both conditions. In summary, this study has revealed the molecular signature of ovarian GCTs in a mouse model that harbors the constitutive activation of TGFBR1. The findings may be further exploited to understand the pathogenesis of a class of poorly defined ovarian tumors.
Keywords: TGFBR1; gene ontology; granulosa cell tumors; signaling pathways.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
A novel mouse model of testicular granulosa cell tumors.Mol Hum Reprod. 2018 Jul 1;24(7):343-356. doi: 10.1093/molehr/gay023. Mol Hum Reprod. 2018. PMID: 29788434 Free PMC article.
-
Disruption of postnatal folliculogenesis and development of ovarian tumor in a mouse model with aberrant transforming growth factor beta signaling.Reprod Biol Endocrinol. 2017 Dec 8;15(1):94. doi: 10.1186/s12958-017-0312-z. Reprod Biol Endocrinol. 2017. PMID: 29221447 Free PMC article.
-
Constitutively active transforming growth factor β receptor 1 in the mouse ovary promotes tumorigenesis.Oncotarget. 2016 Jul 5;7(27):40904-40918. doi: 10.18632/oncotarget.10149. Oncotarget. 2016. PMID: 27344183 Free PMC article.
-
SMAD3 Activation: A Converging Point of Dysregulated TGF-Beta Superfamily Signaling and Genetic Aberrations in Granulosa Cell Tumor Development?Biol Reprod. 2016 Nov;95(5):105. doi: 10.1095/biolreprod.116.143412. Epub 2016 Sep 28. Biol Reprod. 2016. PMID: 27683263 Free PMC article. Review.
-
Multimodality imaging and genomics of granulosa cell tumors.Abdom Radiol (NY). 2020 Mar;45(3):812-827. doi: 10.1007/s00261-019-02172-3. Abdom Radiol (NY). 2020. PMID: 31410505 Review.
Cited by
-
The prognostic value and immunological role of SULF2 in adrenocortical carcinoma.Heliyon. 2023 Feb 11;9(2):e13613. doi: 10.1016/j.heliyon.2023.e13613. eCollection 2023 Feb. Heliyon. 2023. PMID: 36852051 Free PMC article.
-
CRISPR targeting of FOXL2 c.402C>G mutation reduces malignant phenotype in granulosa tumor cells and identifies anti-tumoral compounds.Mol Oncol. 2025 Apr;19(4):1092-1116. doi: 10.1002/1878-0261.13799. Epub 2025 Jan 8. Mol Oncol. 2025. PMID: 39776254 Free PMC article.
-
Loss of Runx1 Induces Granulosa Cell Defects and Development of Ovarian Tumors in the Mouse.Int J Mol Sci. 2022 Nov 21;23(22):14442. doi: 10.3390/ijms232214442. Int J Mol Sci. 2022. PMID: 36430923 Free PMC article.
-
Lineage tracing of mutant granulosa cells reveals in vivo protective mechanisms that prevent granulosa cell tumorigenesis.Cell Death Differ. 2023 May;30(5):1235-1246. doi: 10.1038/s41418-023-01132-1. Epub 2023 Feb 23. Cell Death Differ. 2023. PMID: 36823373 Free PMC article.
References
-
- Pangas S.A., Li X., Umans L., Zwijsen A., Huylebroeck D., Gutierrez C., Wang D., Martin J.F., Jamin S.P., Behringer R.R., et al. Conditional deletion of smad1 and smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol. Cell. Biol. 2008;28:248–257. doi: 10.1128/MCB.01404-07. - DOI - PMC - PubMed
-
- Edson M.A., Nalam R.L., Clementi C., Franco H.L., Demayo F.J., Lyons K.M., Pangas S.A., Matzuk M.M. Granulosa cell-expressed bmpr1a and bmpr1b have unique functions in regulating fertility but act redundantly to suppress ovarian tumor development. Mol. Endocrinol. 2010;24:1251–1266. doi: 10.1210/me.2009-0461. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

