Comparative Study of the Role of Interepithelial Mucosal Mast Cells in the Context of Intestinal Adenoma-Carcinoma Progression
- PMID: 35565377
- PMCID: PMC9105816
- DOI: 10.3390/cancers14092248
Comparative Study of the Role of Interepithelial Mucosal Mast Cells in the Context of Intestinal Adenoma-Carcinoma Progression
Abstract
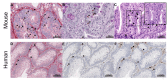
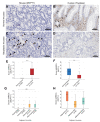
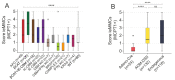
Mast cells (MCs) are crucial players in the relationship between the tumor microenvironment (TME) and cancer cells and have been shown to influence angiogenesis and progression of human colorectal cancer (CRC). However, the role of MCs in the TME is controversially discussed as either pro- or anti-tumorigenic. Genetically engineered mouse models (GEMMs) are the most frequently used in vivo models for human CRC research. In the murine intestine there are at least three different MC subtypes: interepithelial mucosal mast cells (ieMMCs), lamina proprial mucosal mast cells (lpMMCs) and connective tissue mast cells (CTMCs). Interepithelial mucosal mast cells (ieMMCs) in (pre-)neoplastic intestinal formalin-fixed paraffin-embedded (FFPE) specimens of mouse models (total lesions n = 274) and human patients (n = 104) were immunohistochemically identified and semiquantitatively scored. Scores were analyzed along the adenoma-carcinoma sequence in humans and 12 GEMMs of small and large intestinal cancer. The presence of ieMMCs was a common finding in intestinal adenomas and carcinomas in mice and humans. The number of ieMMCs decreased in the course of colonic adenoma-carcinoma sequence in both species (p < 0.001). However, this dynamic cellular state was not observed for small intestinal murine tumors. Furthermore, ieMMC scores were higher in GEMMs with altered Wnt signaling (active β-catenin) than in GEMMs with altered MAPK signaling and wildtypes (WT). In conclusion, we hypothesize that, besides stromal MCs (lpMMCs/CTMCs), particularly the ieMMC subset is important for onset and progression of intestinal neoplasia and may interact with the adjacent neoplastic epithelial cells in dependence on the molecular environment. Moreover, our study indicates the need for adequate GEMMs for the investigation of the intestinal immunologic TME.
Keywords: adenoma-carcinoma sequence; colorectal cancer; genetically engineered mouse models; human; interepithelial mucosal mast cells; tumor microenvironment.
Conflict of interest statement
W.W. has attended Advisory Boards and served as a speaker for Roche, MSD, BMS, AstraZeneca, Pfizer, Merck, Lilly, Boehringer, Novartis, Takeda, Bayer, Amgen, Astellas, Eisai, Illumina, Siemens, Agilent, ADC, GSK, and Molecular Health. W.W. receives research funding from Roche, MSD, BMS, and AstraZeneca. K.S. is consultant for Roche Pharma AG, member of the advisory board of TRIMT GmbH and has filed a patent on a radiopharmaceutical. All other authors declare no conflicts of interest.
Figures





Similar articles
-
Globule Leukocytes and Other Mast Cells in the Mouse Intestine.Vet Pathol. 2018 Jan;55(1):76-97. doi: 10.1177/0300985817705174. Epub 2017 May 11. Vet Pathol. 2018. PMID: 28494703 Free PMC article.
-
Mast Cells Infiltrating Inflamed or Transformed Gut Alternatively Sustain Mucosal Healing or Tumor Growth.Cancer Res. 2015 Sep 15;75(18):3760-70. doi: 10.1158/0008-5472.CAN-14-3767. Epub 2015 Jul 23. Cancer Res. 2015. PMID: 26206557
-
Activation of mucosal mast cells promotes inflammation-related colon cancer development through recruiting and modulating inflammatory CD11b(+)Gr1(+) cells.Cancer Lett. 2015 Aug 10;364(2):173-80. doi: 10.1016/j.canlet.2015.05.014. Epub 2015 May 15. Cancer Lett. 2015. PMID: 25986744
-
Focus on mast cells in the tumor microenvironment: Current knowledge and future directions.Biochim Biophys Acta Rev Cancer. 2023 Jan;1878(1):188845. doi: 10.1016/j.bbcan.2022.188845. Epub 2022 Dec 5. Biochim Biophys Acta Rev Cancer. 2023. PMID: 36476563 Review.
-
Priceless GEMMs: genetically engineered mouse models for colorectal cancer drug development.Trends Pharmacol Sci. 2012 Aug;33(8):449-55. doi: 10.1016/j.tips.2012.05.001. Epub 2012 Jun 26. Trends Pharmacol Sci. 2012. PMID: 22739258 Review.
Cited by
-
Unlocking the Potential of the Human Microbiome for Identifying Disease Diagnostic Biomarkers.Diagnostics (Basel). 2022 Jul 19;12(7):1742. doi: 10.3390/diagnostics12071742. Diagnostics (Basel). 2022. PMID: 35885645 Free PMC article. Review.
-
High Intraepithelial Mast Cell Density in Warthin's Tumor.In Vivo. 2024 May-Jun;38(3):1104-1111. doi: 10.21873/invivo.13544. In Vivo. 2024. PMID: 38688595 Free PMC article.
-
The Controversial Role of Intestinal Mast Cells in Colon Cancer.Cells. 2023 Jan 31;12(3):459. doi: 10.3390/cells12030459. Cells. 2023. PMID: 36766801 Free PMC article. Review.
-
Lysophosphatidylinositol Promotes Chemotaxis and Cytokine Synthesis in Mast Cells with Differential Participation of GPR55 and CB2 Receptors.Int J Mol Sci. 2023 Mar 28;24(7):6316. doi: 10.3390/ijms24076316. Int J Mol Sci. 2023. PMID: 37047288 Free PMC article.
References
-
- Yodavudh S., Tangjitgamol S., Puangsa-art S. Prognostic significance of microvessel density and mast cell density for the survival of Thai patients with primary colorectal cancer. J. Med. Assoc. Thai. 2008;91:723–732. - PubMed
Grants and funding
- 322359157 - FOR2599/Deutsche Forschungsgemeinschaft
- ERC-2016-STG-71518/ERC_/European Research Council/International
- 210592381 - SFB 1054/Deutsche Forschungsgemeinschaft
- 360372040 - SFB 1335/Deutsche Forschungsgemeinschaft
- 395357507 - SFB 1371/Deutsche Forschungsgemeinschaft
- 369799452 - TRR 237/Deutsche Forschungsgemeinschaft
- 452881907 - TRR 338/Deutsche Forschungsgemeinschaft
- 435874434 - RTG 2668, RU 695/9-1, RU 695/12-1/Deutsche Forschungsgemeinschaft
- European Union's Horizon 2020 research and innovative program (grant agreement No 834154)/ERC_/European Research Council/International
- Promotionsförderung/Cusanuswerk, Bischöfliche Studienförderung, Bonn, Germany
LinkOut - more resources
Full Text Sources

