Development and Characterization of MYB-NFIB Fusion Expression in Adenoid Cystic Carcinoma
- PMID: 35565392
- PMCID: PMC9103462
- DOI: 10.3390/cancers14092263
Development and Characterization of MYB-NFIB Fusion Expression in Adenoid Cystic Carcinoma
Abstract
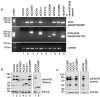
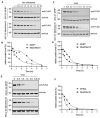
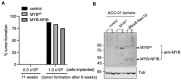
Adenoid cystic carcinoma (ACC) is the second most common cancer type arising from the salivary gland. The frequent occurrence of chromosome t(6;9) translocation leading to the fusion of MYB and NFIB transcription factor genes is considered a genetic hallmark of ACC. This inter-chromosomal rearrangement may encode multiple variants of functional MYB-NFIB fusion in ACC. However, the lack of an ACC model that harbors the t(6;9) translocation has limited studies on defining the potential function and implication of chimeric MYB-NFIB protein in ACC. This report aims to establish a MYB-NFIB fusion protein expressing system in ACC cells for in vitro and in vivo studies. RNA-seq data from MYB-NFIB translocation positive ACC patients' tumors and MYB-NFIB fusion transcript in ACC patient-derived xenografts (ACCX) was analyzed to identify MYB breakpoints and their frequency of occurrence. Based on the MYB breakpoint identified, variants of MYB-NFIB fusion expression system were developed in a MYB-NFIB deficient ACC cell lines. Analysis confirmed MYB-NFIB fusion protein expression in ACC cells and ACCXs. Furthermore, recombinant MYB-NFIB fusion displayed sustained protein stability and impacted transcriptional activities of interferon-associated genes set as compared to a wild type MYB. In vivo tumor formation analysis indicated the capacity of MYB-NFIB fusion cells to grow as implanted tumors, although there were no fusion-mediated growth advantages. This expression system may be useful not only in studies to determine the functional aspects of MYB-NFIB fusion but also in evaluating effective drug response in vitro and in vivo settings.
Keywords: 9) translocation; MYB; MYB-NFIB fusion; adenoid cystic carcinoma; chromosomal t(6.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Young A., Okuyemi O.T. Malignant Salivary Gland Tumors. StatPearls; Treasure Island, FL, USA: 2022. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

