mRNA Subtype of Cancer-Associated Fibroblasts Significantly Affects Key Characteristics of Head and Neck Cancer Cells
- PMID: 35565415
- PMCID: PMC9102192
- DOI: 10.3390/cancers14092286
mRNA Subtype of Cancer-Associated Fibroblasts Significantly Affects Key Characteristics of Head and Neck Cancer Cells
Abstract
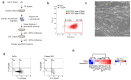
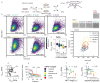
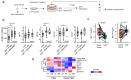
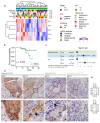
Head and neck squamous cell carcinomas (HNSCC) belong among severe and highly complex malignant diseases showing a high level of heterogeneity and consequently also a variance in therapeutic response, regardless of clinical stage. Our study implies that the progression of HNSCC may be supported by cancer-associated fibroblasts (CAFs) in the tumour microenvironment (TME) and the heterogeneity of this disease may lie in the level of cooperation between CAFs and epithelial cancer cells, as communication between CAFs and epithelial cancer cells seems to be a key factor for the sustained growth of the tumour mass. In this study, we investigated how CAFs derived from tumours of different mRNA subtypes influence the proliferation of cancer cells and their metabolic and biomechanical reprogramming. We also investigated the clinicopathological significance of the expression of these metabolism-related genes in tissue samples of HNSCC patients to identify a possible gene signature typical for HNSCC progression. We found that the right kind of cooperation between cancer cells and CAFs is needed for tumour growth and progression, and only specific mRNA subtypes can support the growth of primary cancer cells or metastases. Specifically, during coculture, cancer cell colony supporting effect and effect of CAFs on cell stiffness of cancer cells are driven by the mRNA subtype of the tumour from which the CAFs are derived. The degree of colony-forming support is reflected in cancer cell glycolysis levels and lactate shuttle-related transporters.
Keywords: HNSCC; cancer; cancer-associated fibroblasts; cell stiffness; tumour microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Stewart B.W., Wild C.P. World Cancer Report 2014. WHO Press; Geneva, Switzerland: 2014.
-
- Kumar D., New J., Vishwakarma V., Joshi R., Enders J., Lin F., Dasari S., Gutierrez W.R., Leef G., Ponnurangam S., et al. Cancer-Associated Fibroblasts Drive Glycolysis in a Targetable Signaling Loop Implicated in Head and Neck Squamous Cell Carcinoma Progression. Cancer Res. 2018;78:3769–3782. doi: 10.1158/0008-5472.CAN-17-1076. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

