The Effect of Ghrelin on the Maturation of Sheep Oocytes and Early Embryonic Development In Vitro
- PMID: 35565584
- PMCID: PMC9100601
- DOI: 10.3390/ani12091158
The Effect of Ghrelin on the Maturation of Sheep Oocytes and Early Embryonic Development In Vitro
Abstract
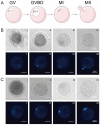
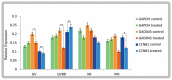
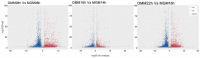
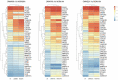
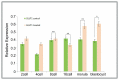
In vitro maturation (IVM) of sheep oocytes and early embryonic development are of great scientific importance for the study of reproductive development in sheep. Ghrelin is an important hormone that regulates the secretion of the growth hormone (GH). In this study, different gradients of ghrelin (0, 100, 200, and 300 ng/mL) were added to the IVM system of sheep oocytes to observe their cell morphology, and Hosesth 33342 staining was used to determine the time taken for oocytes to reach different developmental stages. We found 200 ng/mL ghrelin to be the optimal concentration. The RNA-seq analysis showed that many signaling pathways were significantly altered by ghrelin. Cell cycle, Wnt, and oxidative phosphorylation were activated; the P53 was inhibited. These pathways together regulate the maturation of oocytes and early embryonic development in vitro. The effects of the addition of ghrelin were verified by the expression of GLUT1 in early embryonic development. The results suggest that adding ghrelin shortens the duration of the IVM of sheep oocytes and hinders early embryonic development. This study provides new insights into the effects of exogenous ghrelin on sheep oocyte maturation and early embryonic development in vitro.
Keywords: RNA-seq; cell cycle; early embryo; ghrelin; sheep oocyte.
Conflict of interest statement
Authors declare that there is no conflict of interest.
Figures


Similar articles
-
Effects of ghrelin on developmental competence and gene expression of in vitro fertilized ovine embryos.Theriogenology. 2013 Mar 1;79(4):695-701. doi: 10.1016/j.theriogenology.2012.11.026. Epub 2013 Jan 2. Theriogenology. 2013. PMID: 23290751
-
A pre-in vitro maturation medium containing cumulus oocyte complex ligand-receptor signaling molecules maintains meiotic arrest, supports the cumulus oocyte complex and improves oocyte developmental competence.Mol Hum Reprod. 2017 Sep 1;23(9):594-606. doi: 10.1093/molehr/gax032. Mol Hum Reprod. 2017. PMID: 28586460
-
The presence of acylated ghrelin during in vitro maturation of bovine oocytes induces cumulus cell DNA damage and apoptosis, and impairs early embryo development.Zygote. 2017 Oct;25(5):601-611. doi: 10.1017/S0967199417000478. Epub 2017 Sep 20. Zygote. 2017. PMID: 28929981
-
Hormonal actions during oocyte maturation influence fertilization and early embryonic development.Ann N Y Acad Sci. 1991;626:137-58. doi: 10.1111/j.1749-6632.1991.tb37908.x. Ann N Y Acad Sci. 1991. PMID: 2058950 Review.
-
Oocyte maturation. Basic and clinical aspects of in vitro maturation (IVM) with special emphasis of the role of FF-MAS.Dan Med Bull. 2008 Feb;55(1):1-16. Dan Med Bull. 2008. PMID: 18321442 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

