Assessing Genetic Variation in Wild and Domesticated Pikeperch Populations: Implications for Conservation and Fish Farming
- PMID: 35565604
- PMCID: PMC9102197
- DOI: 10.3390/ani12091178
Assessing Genetic Variation in Wild and Domesticated Pikeperch Populations: Implications for Conservation and Fish Farming
Abstract
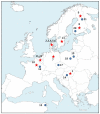
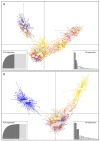
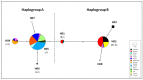
The pikeperch is a freshwater/brackish water fish species with growing interest for European aquaculture. Wild populations show signs of decline in many areas of the species natural range due to human activities. The comparative evaluation of genetic status in wild and domesticated populations is extremely useful for the future establishment of genetic breeding programs. The main objective of the present study was to assess and compare the genetic variability of 13 domesticated populations from commercial farms and 8 wild populations, developing an efficient microsatellite multiplex tool for genotyping. Partial cytochrome b gene sequences were also used to infer phylogeographic relationships. Results show that on average, the domesticated populations do not exhibit significantly lower levels of genetic diversity compared to the wild ones and do not suffer from inbreeding. Nuclear data provide evidence that pikeperch populations in Europe belong to at least two genetically differentiated groups: the first one is predominantly present in Northern Europe and around the Baltic Sea, while the second one comprises populations from Central Europe. In this second group, Hungarian origin populations constitute a differentiated stock that needs special consideration. Aquaculture broodstocks analyzed appear to contain fish of a single origin with only a few exceptions.
Keywords: Sander lucioperca; aquaculture; cyt b; differentiation; microsatellites.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Kestemont P., Dabrowski K., Summerfelt R.C. In: Biology and Culture of Percid Fishes: Principles and Practices. Kestemont P., Summerfelt R.C., Dabrowski K., editors. Springer; Dordrecht, The Netherlands: 2015.
-
- Haponski A.E., Stepien C.A. Phylogenetic and biogeographical relationships of the sander pikeperches (percidae: Perciformes): Patterns across North America and Eurasia. Biol. J. Linn. Soc. 2013;110:156–179. doi: 10.1111/bij.12114. - DOI
-
- Eschbach E., Nolte A.W., Kohlmann K., Kersten P., Kail J., Arlinghaus R. Population differentiation of zander (Sander lucioperca) across native and newly colonized ranges suggests increasing admixture in the course of an invasion. Evol. Appl. 2014;7:555–568. doi: 10.1111/eva.12155. - DOI - PMC - PubMed
-
- FAO . Global Capture Production. FAO FishStat, Fisheries and Aquaculture Department; Electronic Database: 2019.
Grants and funding
LinkOut - more resources
Full Text Sources

