Acute Stress in Lesser-Spotted Catshark (Scyliorhinus canicula Linnaeus, 1758) Promotes Amino Acid Catabolism and Osmoregulatory Imbalances
- PMID: 35565621
- PMCID: PMC9105869
- DOI: 10.3390/ani12091192
Acute Stress in Lesser-Spotted Catshark (Scyliorhinus canicula Linnaeus, 1758) Promotes Amino Acid Catabolism and Osmoregulatory Imbalances
Abstract
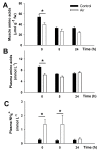
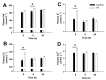
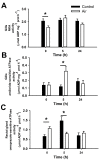
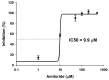
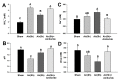
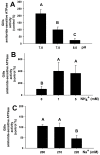
Acute-stress situations in vertebrates induce a series of physiological responses to cope with the event. While common secondary stress responses include increased catabolism and osmoregulatory imbalances, specific processes depend on the taxa. In this sense, these processes are still largely unknown in ancient vertebrates such as marine elasmobranchs. Thus, we challenged the lesser spotted catshark (Scyliorhinus canicula) to 18 min of air exposure, and monitored their recovery after 0, 5, and 24 h. This study describes amino acid turnover in the liver, white muscle, gills, and rectal gland, and plasma parameters related to energy metabolism and osmoregulatory imbalances. Catsharks rely on white muscle amino acid catabolism to face the energy demand imposed by the stressor, producing NH4+. While some plasma ions (K+, Cl- and Ca2+) increased in concentration after 18 min of air exposure, returning to basal values after 5 h of recovery, Na+ increased after just 5 h of recovery, coinciding with a decrease in plasma NH4+. These changes were accompanied by increased activity of a branchial amiloride-sensitive ATPase. Therefore, we hypothesize that this enzyme may be a Na+/H+ exchanger (NHE) related to NH4+ excretion. The action of an omeprazole-sensitive ATPase, putatively associated to a H+/K+-ATPase (HKA), is also affected by these allostatic processes. Some complementary experiments were carried out to delve a little deeper into the possible branchial enzymes sensitive to amiloride, including in vivo and ex vivo approaches, and partial sequencing of a nhe1 in the gills. This study describes the possible presence of an HKA enzyme in the rectal gland, as well as a NHE in the gills, highlighting the importance of understanding the relationship between acute stress and osmoregulation in elasmobranchs.
Keywords: NHE; Scyliorhinus canicula; air exposure; amiloride; amino acid consumption; elasmobranchs; osmoregulation; stress.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Iwama G.K. Stress in fish. Ann. N. Y. Acad. Sci. 1998;851:304–310. doi: 10.1111/j.1749-6632.1998.tb09005.x. - DOI
-
- Wedemeyer G.A., Barton B.A., McLeay D.J. Stress and acclimation. In: Schreck C.B., Moyle P.B., editors. Methods of Fish Biology. American Fisheries Society; Bethesda, MD, USA: 1990. pp. 451–489.
-
- Dickens M.J., Delehanty D.J., Romero L.M. Stress: An inevitable component of animal translocation. Biol. Conserv. 2010;143:1329–1341. doi: 10.1016/j.biocon.2010.02.032. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

