Walnut Oil Reduces Aβ Levels and Increases Neurite Length in a Cellular Model of Early Alzheimer Disease
- PMID: 35565661
- PMCID: PMC9099939
- DOI: 10.3390/nu14091694
Walnut Oil Reduces Aβ Levels and Increases Neurite Length in a Cellular Model of Early Alzheimer Disease
Abstract
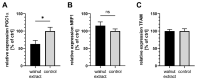
(1) Background: Mitochondria are the cells' main source of energy. Mitochondrial dysfunction represents a key hallmark of aging and is linked to the development of Alzheimer's disease (AD). Maintaining mitochondrial function might contribute to healthy aging and the prevention of AD. The Mediterranean diet, including walnuts, seems to prevent age-related neurodegeneration. Walnuts are a rich source of α-linolenic acid (ALA), an essential n3-fatty acid and the precursor for n3-long-chain polyunsaturated fatty acids (n3-PUFA), which might potentially improve mitochondrial function. (2) Methods: We tested whether a lipophilic walnut extract (WE) affects mitochondrial function and other parameters in human SH-SY5Y cells transfected with the neuronal amyloid precursor protein (APP695). Walnut lipids were extracted using a Soxhlet Extraction System and analyzed using GC/MS and HPLC/FD. Adenosine triphosphate (ATP) concentrations were quantified under basal conditions in cell culture, as well as after rotenone-induced stress. Neurite outgrowth was investigated, as well as membrane integrity, cellular reactive oxygen species, cellular peroxidase activity, and citrate synthase activity. Beta-amyloid (Aβ) was quantified using homogenous time-resolved fluorescence. (3) Results: The main constituents of WE are linoleic acid, oleic acid, α-linolenic acid, and γ- and δ-tocopherol. Basal ATP levels following rotenone treatment, as well as citrate synthase activity, were increased after WE treatment. WE significantly increased cellular reactive oxygen species but lowered peroxidase activity. Membrane integrity was not affected. Furthermore, WE treatment reduced Aβ1-40 and stimulated neurite growth. (4) Conclusions: WE might increase ATP production after induction of mitochondrial biogenesis. Decreased Aβ1-40 formation and enhanced ATP levels might enhance neurite growth, making WE a potential agent to enhance neuronal function and to prevent the development of AD. In this sense, WE could be a promising agent for the prevention of AD.
Keywords: PUFA; aging; mitochondria; neurodegeneration; poly-unsaturated fatty acids; vitamin E; walnut.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Redox Active α-Lipoic Acid Differentially Improves Mitochondrial Dysfunction in a Cellular Model of Alzheimer and Its Control Cells.Int J Mol Sci. 2022 Aug 16;23(16):9186. doi: 10.3390/ijms23169186. Int J Mol Sci. 2022. PMID: 36012451 Free PMC article.
-
Effects of Urolithin A on Mitochondrial Parameters in a Cellular Model of Early Alzheimer Disease.Int J Mol Sci. 2021 Aug 3;22(15):8333. doi: 10.3390/ijms22158333. Int J Mol Sci. 2021. PMID: 34361099 Free PMC article.
-
Comparative Effects of Alpha- and Gamma-Tocopherol on Mitochondrial Functions in Alzheimer's Disease In Vitro Model.Sci Rep. 2020 Jun 2;10(1):8962. doi: 10.1038/s41598-020-65570-4. Sci Rep. 2020. PMID: 32488024 Free PMC article.
-
Beneficial Effects of Walnuts on Cognition and Brain Health.Nutrients. 2020 Feb 20;12(2):550. doi: 10.3390/nu12020550. Nutrients. 2020. PMID: 32093220 Free PMC article. Review.
-
Olesoxime improves cerebral mitochondrial dysfunction and enhances Aβ levels in preclinical models of Alzheimer's disease.Exp Neurol. 2020 Jul;329:113286. doi: 10.1016/j.expneurol.2020.113286. Epub 2020 Mar 18. Exp Neurol. 2020. PMID: 32199815 Review.
Cited by
-
Neuroprotective effects of walnut (Juglans regia L.) in nervous system disorders: A comprehensive review.Iran J Basic Med Sci. 2024;27(12):1492-1505. doi: 10.22038/ijbms.2024.79854.17302. Iran J Basic Med Sci. 2024. PMID: 39539440 Free PMC article. Review.
-
Impact of Nut Consumption on Cognition across the Lifespan.Nutrients. 2023 Feb 16;15(4):1000. doi: 10.3390/nu15041000. Nutrients. 2023. PMID: 36839359 Free PMC article. Review.
-
Impaired autophagy in amyloid-beta pathology: A traditional review of recent Alzheimer's research.J Biomed Res. 2022 Sep 28;37(1):30-46. doi: 10.7555/JBR.36.20220145. J Biomed Res. 2022. PMID: 36642915 Free PMC article.
-
Influence of the Extraction Method on the Quality and Chemical Composition of Walnut (Juglans regia L.) Oil.Molecules. 2022 Nov 8;27(22):7681. doi: 10.3390/molecules27227681. Molecules. 2022. PMID: 36431782 Free PMC article.
-
Caenorhabditis elegans as a Model for the Effects of Phytochemicals on Mitochondria and Aging.Biomolecules. 2022 Oct 24;12(11):1550. doi: 10.3390/biom12111550. Biomolecules. 2022. PMID: 36358900 Free PMC article. Review.
References
-
- Gutierrez L., Folch A., Rojas M., Cantero J.L., Atienza M., Folch J., Camins A., Ruiz A., Papandreou C., Bulló M. Effects of Nutrition on Cognitive Function in Adults with or without Cognitive Impairment: A Systematic Review of Randomized Controlled Clinical Trials. Nutrients. 2021;13:3728. doi: 10.3390/nu13113728. - DOI - PMC - PubMed
-
- Valls-Pedret C., Lamuela-Raventós R.M., Medina-Remón A., Quintana M., Corella D., Pintó X., Martínez-González M.Á., Estruch R., Ros E. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J. Alzheimer’s Dis. JAD. 2012;29:773–782. doi: 10.3233/JAD-2012-111799. - DOI - PubMed
-
- Valls-Pedret C., Sala-Vila A., Serra-Mir M., Corella D., de La Torre R., Martínez-González M.Á., Martínez-Lapiscina E.H., Fitó M., Pérez-Heras A., Salas-Salvadó J., et al. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern. Med. 2015;175:1094–1103. doi: 10.1001/jamainternmed.2015.1668. - DOI - PubMed
-
- Martínez-Lapiscina E.H., Clavero P., Toledo E., Estruch R., Salas-Salvadó J., San Julián B., Sanchez-Tainta A., Ros E., Valls-Pedret C., Martinez-Gonzalez M.Á. Mediterranean diet improves cognition: The PREDIMED-NAVARRA randomised trial. J Neurol. Neurosurg. Psychiatry. 2013;84:1318–1325. doi: 10.1136/jnnp-2012-304792. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

