Effects of Classic Ketogenic Diet in Children with Refractory Epilepsy: A Retrospective Cohort Study in Kingdom of Bahrain
- PMID: 35565714
- PMCID: PMC9105742
- DOI: 10.3390/nu14091744
Effects of Classic Ketogenic Diet in Children with Refractory Epilepsy: A Retrospective Cohort Study in Kingdom of Bahrain
Abstract
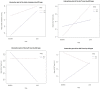
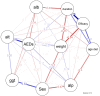
Background: The classic ketogenic diet (cKD) has been used worldwide as an effective therapy for children with drug-resistant epilepsy. However, there have been no studies performed in Middle Eastern countries in order to assess the efficacy, side effects, predictors of cKD response and factors mostly associated with diet adherence. This study aims to assess the efficacy of cKD ratios of 4:1 and 3:1 and their influence on growth and biochemical parameters, particularly lipid profile and liver function tests (LFTs), and the factors most associated with diet adherence in a cohort of children with drug-resistant epilepsy in Bahrain. Methods: Baseline and follow-up data related to patients’ demographic and biochemical variables, epilepsy episodes, diet history and anthropometric measurements were retrieved for a total of 24 children treated with cKD in Bahrain. Results: After 6 months cKD initiation, 58.3% were positive responders with >50% seizure rate reduction, and 33.3% became seizure-free at 12 months. After 6 months of intervention with cKD, the level of triglycerides and albumin had a significant (p < 0.05) average increase over time of +1.47 mmol/L and 4.3 g/L, respectively. Although the median values of total cholesterol and alanine transaminase increased, respectively, following cKD initiation, the difference over time was not statistically significant. The mean z-scores for weight, height, and body mass index (or weight-for-length) did not change significantly at 12 months follow-up. cKD duration was the highest correlated variable with cKD efficacy (r = 0.76), which was followed by age at cKD initiation (r = 0.47). The cKD was discontinued by 14 patients (58.3%) during the first follow-up period (6 months), which was mainly due to inefficacy (n = 8), poor compliance (n = 3), food refusal (n = 1), achieved required efficacy (n = 1) and death (n = 1). Conclusions: cKD is an effective treatment for patients with drug-resistant epilepsy, and positive response to cKD was the main factor that increased adherence to the diet. Although long-term cKD could increase the risk of dyslipidemia and hepatic problems, it appears safe for children. Consequently, close monitoring and emphasis on healthy fats is of high priority.
Keywords: diet therapy; ketogenic diet; pediatric epilepsy; seizures.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- World Health Organization Epilepsy. Key Facts. [(accessed on 23 January 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy.
-
- Kossoff E.H., Zupec-Kania B.A., Auvin S., Ballaban-Gil K.R., Christina Bergqvist A.G., Blackford R., Buchhalter J.R., Caraballo R.H., Cross J.H., Dahlin M.G., et al. Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open. 2018;3:175–192. doi: 10.1002/epi4.12225. - DOI - PMC - PubMed
-
- Wirrell E.C., Grossardt B., Wong-Kisiel L.C., Nickels K.C. Incidence and classification of new-onset epilepsy and epilepsy syndromes in children in Olmsted County, Minnesota from 1980 to 2004: A population-based study. Epilepsy Res. 2011;95:110–118. doi: 10.1016/j.eplepsyres.2011.03.009. - DOI - PMC - PubMed
-
- De Kinderen R.J.A., Lambrechts D.A.J.E., Wijnen B.F.M., Postulart D., Aldenkamp A.P., Majoie M.H.J.M., Evers S.M.A.A. An economic evaluation of the ketogenic diet versus care as usual in children and adolescents with intractable epilepsy: An interim analysis. Epilepsia. 2016;57:41–50. doi: 10.1111/epi.13254. - DOI - PubMed