Chronic Circadian Disruption and Sleep Restriction Influence Subjective Hunger, Appetite, and Food Preference
- PMID: 35565768
- PMCID: PMC9105437
- DOI: 10.3390/nu14091800
Chronic Circadian Disruption and Sleep Restriction Influence Subjective Hunger, Appetite, and Food Preference
Abstract
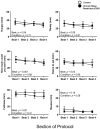
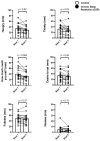
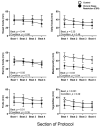
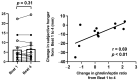
Chronic circadian disruption (CCD), such as occurs during rotating shiftwork, and insufficient sleep are each independently associated with poor health outcomes, including obesity and glucose intolerance. A potential mechanism for poor health is increased energy intake (i.e., eating), particularly during the circadian night, when the physiological response to energy intake is altered. However, the contributions of CCD and insufficient sleep to subjective hunger, appetite, food preference, and appetitive hormones are not clear. To disentangle the influences of these factors, we studied seventeen healthy young adults in a 32-day in-laboratory study designed to distribute sleep, wakefulness, and energy intake equally across all phases of the circadian cycle, thereby imposing CCD. Participants were randomized to the Control (1:2 sleep:wake ratio, n = 8) or chronic sleep restriction (CSR, 1:3.3 sleep:wake ratio, n = 9) conditions. Throughout each waking episode the participants completed visual analog scales pertaining to hunger, appetite, and food preference. A fasting blood sample was collected to assess appetitive hormones. CCD was associated with a significant decrease in hunger and appetite in a multitude of domains in both the Control and CSR groups. This change in hunger was significantly correlated with changes in the ghrelin/leptin ratio. These findings further our understanding of the contributions of CCD and insufficient sleep on subjective hunger and appetite as well as of their possible contributions to adverse health behaviors.
Keywords: circadian misalignment; ghrelin; insufficient sleep; leptin; shiftwork.
Conflict of interest statement
A.W.M. declares consultancy for Somni Corporation; J.T.H. declares no conflict; E.B.K. declares travel support from the American Academy of Sleep Medicine Foundation, Gordon Research Conference, Sleep Research Society, Santa Fe institute, DGSM (German Sleep Society); consultancy for Circadian Therapeutics, National Sleep Foundation, Puerto Rico Science Technology Trust, Sanofi-Genzyme, Yale University Press; partner owns Chronsulting. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Liu Q., Shi J., Duan P., Liu B., Li T., Wang C., Li H., Yang T., Gan Y., Wang X., et al. Is shift work associated with a higher risk of overweight or obesity? A systematic review of observational studies with meta-analysis. Int. J. Epidemiol. 2018;47:1956–1971. doi: 10.1093/ije/dyy079. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21HD086392/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- T32HL007901, K01HL146992, R56HL156948, K24HL105664, R01HL114088, R01HL128538,/National Heart Lung and Blood Institute
- R01 HD107064/HD/NICHD NIH HHS/United States
- P01AG009975/AG/NIA NIH HHS/United States
- HFP02802, HFP04201, HDP0006/National Space Biomedical Research Institute
LinkOut - more resources
Full Text Sources

