A Nutritional Approach for the Management of Chemotherapy-Induced Diarrhea in Patients with Colorectal Cancer
- PMID: 35565769
- PMCID: PMC9100930
- DOI: 10.3390/nu14091801
A Nutritional Approach for the Management of Chemotherapy-Induced Diarrhea in Patients with Colorectal Cancer
Abstract
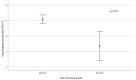
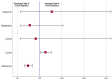
This study aimed to determine if dietary modifications using a nutritional regimen could prevent or reduce the incidence of cancer therapy-induced diarrhea in patients with metastatic colorectal cancer and to evaluate the relationship of Vitamin D blood levels with diarrhea severity. Patients with metastatic colorectal cancer were enrolled. A Mediterranean diet, containing some special limitations aiming to reduce the risk of diarrhea, was administered before and during the entire chemotherapy program. Enrolled patients numbering 60/137 (44%) had diarrhea during chemotherapy. Adherence to the diet was high in 36 (26.3%) patients, medium in 94 (68.6%), and low in 7 (5.1%). Mean adherence to the diet was significantly lower in patients who experienced diarrhea with maximum grade 2−3 compared to those who had no diarrhea or grade 1 diarrhea (score = 5.4 ± 1.9 vs. 7.1 ± 1.5, p < 0.001). Patients with higher adherence to the diet had a lower risk of grade 2−3 diarrhea (odds ratio: 0.5 (95% CI: 0.3−0.7, p < 0.001)). In addition, patients who completed a higher number of chemotherapy cycles had an increased risk of grade 2−3 diarrhea (odds ratio: 1.2 (95% CI: 1.0−1.5, p = 0.02)). Of note, a lower level of Vitamin D correlated with an increased risk of G2-G3 diarrhea (p = 0.03). A diet based on vegetables with a controlled fiber content, Mediterranean Modified Healthy Diet (MMHD), is useful to control the incidence of cancer therapy-induced diarrhea.
Keywords: Vitamin D; cancer therapy-induced diarrhea; colorectal cancer; diet.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Miroddi M., Sterrantino C., Simonelli I., Ciminata G., Phillips R.S., Calapai G. Risk of grade 3-4 diarrhea and mucositis in colorectal cancer patients receiving anti-EGFR monoclonal antibodies regimens: A meta-analysis of 18 randomized controlled clinical trials. Crit. Rev. Oncol. Hematol. 2015;96:355–371. doi: 10.1016/j.critrevonc.2015.06.004. - DOI - PubMed
-
- Douillard J.Y., Cunningham D., Roth A.D., Navarro M., James R.D., Karasek P., Jandik P., Iveson T., Carmichael J., Alakl M., et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/S0140-6736(00)02034-1. - DOI - PubMed
-
- Saltz L.B., Cox J.V., Blanke C., Rosen L.S., Fehrenbacher L., Moore M.J., Maroun J.A., Ackland S.P., Locker P.K., Pirotta N., et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N. Engl. J. Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. - DOI - PubMed
-
- Tournigand C., André T., Achille E., Lledo G., Flesh M., Mery-Mignard D., Quinaux E., Couteau C., Buyse M., Ganem G., et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J. Clin. Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical