Prenatal Choline Supplementation Alters One Carbon Metabolites in a Rat Model of Periconceptional Alcohol Exposure
- PMID: 35565848
- PMCID: PMC9100923
- DOI: 10.3390/nu14091874
Prenatal Choline Supplementation Alters One Carbon Metabolites in a Rat Model of Periconceptional Alcohol Exposure
Abstract
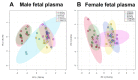
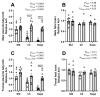
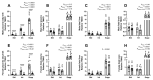
Prenatal alcohol exposure disturbs fetal and placental growth and can alter DNA methylation (DNAm). Supplementation with the methyl donor choline can increase fetal and placental growth and restore DNAm, suggesting converging effects on one-carbon metabolism (1CM). We investigated the impact of periconceptional ethanol (PCE) exposure and prenatal choline supplementation on 1CM in maternal, placental, and fetal compartments. Female Sprague Dawley rats were given a liquid diet containing 12.5% ethanol (PCE) or 0% ethanol (control) for 4 days before and 4 days after conception. Dams were then placed on chow with different concentrations of choline (1.6 g, 2.6 g, or 7.2 g choline/kg chow). Plasma and tissues were collected in late gestation for the analysis of 1CM components by means of mass spectrometry and real-time PCR. PCE reduced placental components of 1CM, particularly those relating to folate metabolism, resulting in a 3−7.5-fold reduction in the ratio of s-adenosylmethionine:s-adenosylhomocysteine (SAM:SAH) (p < 0.0001). Choline supplementation increased placental 1CM components and the SAM:SAH ratio (3.5−14.5-fold, p < 0.0001). In the maternal and fetal compartments, PCE had little effect, whereas choline increased components of 1CM. This suggests that PCE impairs fetal development via altered placental 1CM, highlighting its role in modulating nutritional inputs to optimize fetal development.
Keywords: DNA methylation; mass spectrometry; maternal nutrition; methyl group; one carbon metabolism; placenta; prenatal alcohol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Patra J., Bakker R., Irving H., Jaddoe V.W., Malini S., Rehm J. Dose-response relationship between alcohol consumption before and during pregnancy and the risks of low birthweight, preterm birth and small for gestational age (SGA)-a systematic review and meta-analyses. BJOG. 2011;118:1411–1421. doi: 10.1111/j.1471-0528.2011.03050.x. - DOI - PMC - PubMed
-
- May P.A., Blankenship J., Marais A.S., Gossage J.P., Kalberg W.O., Barnard R., De Vries M., Robinson L.K., Adnams C.M., Buckley D., et al. Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol. Clin. Exp. Res. 2013;37:818–830. doi: 10.1111/acer.12033. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

