Role of Chrononutrition in the Antihypertensive Effects of Natural Bioactive Compounds
- PMID: 35565887
- PMCID: PMC9103085
- DOI: 10.3390/nu14091920
Role of Chrononutrition in the Antihypertensive Effects of Natural Bioactive Compounds
Abstract
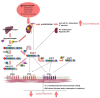
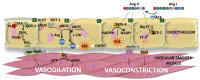
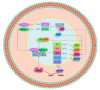
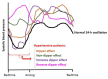
Hypertension (HTN) is one of the main cardiovascular risk factors and is considered a major public health problem. Numerous approaches have been developed to lower blood pressure (BP) in hypertensive patients, most of them involving pharmacological treatments. Within this context, natural bioactive compounds have emerged as a promising alternative to drugs in HTN prevention. This work reviews not only the mechanisms of BP regulation by these antihypertensive compounds, but also their efficacy depending on consumption time. Although a plethora of studies has investigated food-derived compounds, such as phenolic compounds or peptides and their impact on BP, only a few addressed the relevance of time consumption. However, it is known that BP and its main regulatory mechanisms show a 24-h oscillation. Moreover, evidence shows that phenolic compounds can interact with clock genes, which regulate the biological rhythm followed by many physiological processes. Therefore, further research might be carried out to completely elucidate the interactions along the time-nutrition-hypertension axis within the framework of chrononutrition.
Keywords: biological rhythms; blood pressure; hypertension; peptides; phenolic compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
[Profile on circadian blood pressure and the influencing factors in essential hypertensive patients after treatment].Zhonghua Liu Xing Bing Xue Za Zhi. 2004 Aug;25(8):710-4. Zhonghua Liu Xing Bing Xue Za Zhi. 2004. PMID: 15555399 Chinese.
-
Chronotherapy with conventional blood pressure medications improves management of hypertension and reduces cardiovascular and stroke risks.Hypertens Res. 2016 May;39(5):277-92. doi: 10.1038/hr.2015.142. Epub 2015 Dec 10. Hypertens Res. 2016. PMID: 26657008 Review.
-
Effect of Catheter-Based Renal Denervation on Morning and Nocturnal Blood Pressure: Insights From SYMPLICITY HTN-3 and SYMPLICITY HTN-Japan.Hypertension. 2015 Dec;66(6):1130-7. doi: 10.1161/HYPERTENSIONAHA.115.06260. Epub 2015 Oct 5. Hypertension. 2015. PMID: 26558819
-
Sleep-time blood pressure: Unique sensitive prognostic marker of vascular risk and therapeutic target for prevention.Sleep Med Rev. 2017 Jun;33:17-27. doi: 10.1016/j.smrv.2016.04.001. Epub 2016 Apr 14. Sleep Med Rev. 2017. PMID: 27316324 Review.
-
Bedtime administration of long-acting antihypertensive drugs restores normal nocturnal blood pressure fall in nondippers with essential hypertension.Clin Exp Nephrol. 2009 Oct;13(5):467-472. doi: 10.1007/s10157-009-0184-4. Epub 2009 May 16. Clin Exp Nephrol. 2009. PMID: 19449087
Cited by
-
Use of Strawberry Tree (Arbutus unedo) as a Source of Functional Fractions with Biological Activities.Foods. 2022 Nov 28;11(23):3838. doi: 10.3390/foods11233838. Foods. 2022. PMID: 36496646 Free PMC article. Review.
-
An Overview of the Circadian Clock in the Frame of Chronotherapy: From Bench to Bedside.Pharmaceutics. 2022 Jul 6;14(7):1424. doi: 10.3390/pharmaceutics14071424. Pharmaceutics. 2022. PMID: 35890319 Free PMC article. Review.
-
Valorization of Chicken Slaughterhouse Byproducts to Obtain Antihypertensive Peptides.Nutrients. 2023 Jan 15;15(2):457. doi: 10.3390/nu15020457. Nutrients. 2023. PMID: 36678328 Free PMC article. Review.
-
Grape Seed Proanthocyanidins Modulate the Hepatic Molecular Clock via MicroRNAs.Mol Nutr Food Res. 2022 Dec;66(23):e2200443. doi: 10.1002/mnfr.202200443. Epub 2022 Oct 26. Mol Nutr Food Res. 2022. PMID: 36189890 Free PMC article.
References
-
- Unger T., Borghi C., Charchar F., Khan N.A., Poulter N.R., Prabhakaran D., Ramirez A., Schlaich M., Stergiou G.S., Tomaszewski M., et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75:1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

