A Review on the Current State and Future Perspectives of [99mTc]Tc-Housed PSMA-i in Prostate Cancer
- PMID: 35565970
- PMCID: PMC9099988
- DOI: 10.3390/molecules27092617
A Review on the Current State and Future Perspectives of [99mTc]Tc-Housed PSMA-i in Prostate Cancer
Abstract
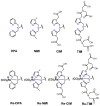
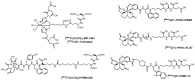
Recently, prostate-specific membrane antigen (PSMA) has gained momentum in tumor nuclear molecular imaging as an excellent target for both the diagnosis and therapy of prostate cancer. Since 2008, after years of preclinical research efforts, a plentitude of radiolabeled compounds mainly based on low molecular weight PSMA inhibitors (PSMA-i) have been described for imaging and theranostic applications, and some of them have been transferred to the clinic. Most of these compounds include radiometals (e.g., 68Ga, 64Cu, 177Lu) for positron emission tomography (PET) imaging or endoradiotherapy. Nowadays, although the development of new PET tracers has caused a significant drop in single-photon emission tomography (SPECT) research programs and the development of new technetium-99m (99mTc) tracers is rare, this radionuclide remains the best atom for SPECT imaging owing to its ideal physical decay properties, convenient availability, and rich and versatile coordination chemistry. Indeed, 99mTc still plays a relevant role in diagnostic nuclear medicine, as the number of clinical examinations based on 99mTc outscores that of PET agents and 99mTc-PSMA SPECT/CT may be a cost-effective alternative for 68Ga-PSMA PET/CT. This review aims to give an overview of the specific features of the developed [99mTc]Tc-tagged PSMA agents with particular attention to [99mTc]Tc-PSMA-i. The chemical and pharmacological properties of the latter will be compared and discussed, highlighting the pros and cons with respect to [68Ga]Ga-PSMA11.
Keywords: PSMA; SPECT; gallium-68; molecular imaging; nanoparticles; prostate cancer; target-specific; technetium-99m.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
-
Diagnostic sensitivity of Tc-99m HYNIC PSMA SPECT/CT in prostate carcinoma: A comparative analysis with Ga-68 PSMA PET/CT.Prostate. 2017 Aug;77(11):1205-1212. doi: 10.1002/pros.23379. Epub 2017 Jun 26. Prostate. 2017. PMID: 28649735
-
Comparison of hybrid 68Ga-PSMA-PET/CT and 99mTc-DPD-SPECT/CT for the detection of bone metastases in prostate cancer patients: Additional value of morphologic information from low dose CT.Eur Radiol. 2018 Feb;28(2):610-619. doi: 10.1007/s00330-017-4994-6. Epub 2017 Aug 4. Eur Radiol. 2018. PMID: 28779400
-
Diagnostic efficacy of [99mTc]Tc-PSMA SPECT/CT for prostate cancer: a meta-analysis.BMC Cancer. 2024 Aug 8;24(1):982. doi: 10.1186/s12885-024-12734-4. BMC Cancer. 2024. PMID: 39118101 Free PMC article.
-
Prostate-specific membrane antigen-directed imaging and radioguided surgery with single-photon emission computed tomography: state of the art and future outlook.Expert Rev Med Devices. 2022 Nov;19(11):815-824. doi: 10.1080/17434440.2022.2146999. Epub 2022 Nov 14. Expert Rev Med Devices. 2022. PMID: 36370108 Review.
Cited by
-
[99mTc]Tc-PSMA-T4-Novel SPECT Tracer for Metastatic PCa: From Bench to Clinic.Molecules. 2022 Oct 25;27(21):7216. doi: 10.3390/molecules27217216. Molecules. 2022. PMID: 36364046 Free PMC article.
-
TrisOxine abiotic siderophores for technetium complexation: radiolabeling and biodistribution studies.EJNMMI Radiopharm Chem. 2023 Oct 19;8(1):32. doi: 10.1186/s41181-023-00214-2. EJNMMI Radiopharm Chem. 2023. PMID: 37856008 Free PMC article.
-
The Theranostic Optimization of PSMA-GCK01 Does Not Compromise the Imaging Characteristics of [99mTc]Tc-PSMA-GCK01 Compared to Dedicated Diagnostic [99mTc]Tc-EDDA/HYNIC-iPSMA in Prostate Cancer.Mol Imaging Biol. 2024 Feb;26(1):81-89. doi: 10.1007/s11307-023-01881-y. Epub 2023 Dec 8. Mol Imaging Biol. 2024. PMID: 38066252 Free PMC article.
-
Molecularly Targeted Lanthanide Nanoparticles for Cancer Theranostic Applications.Nanomaterials (Basel). 2024 Jan 31;14(3):296. doi: 10.3390/nano14030296. Nanomaterials (Basel). 2024. PMID: 38334567 Free PMC article. Review.
-
Development of [99mTc]TcO-ABX474: Design, Synthesis, and Biological Evaluation of PSMA-Binding Technetium-99m Radioligands for SPECT Imaging of Prostate Cancer.J Med Chem. 2025 Apr 10;68(7):7213-7231. doi: 10.1021/acs.jmedchem.4c02767. Epub 2025 Mar 26. J Med Chem. 2025. PMID: 40135799 Free PMC article.
References
-
- Cancer Today. [(accessed on 25 November 2021)]. Available online: http://gco.iarc.fr/today/home.
-
- Schmidkonz C., Cordes M., Beck M., Goetz T.I., Schmidt D., Prante O., Bäuerle T., Uder M., Wullich B., Goebell P., et al. SPECT/CT with the PSMA Ligand 99mTc-MIP-1404 for Whole-Body Primary Staging of Patients with Prostate Cancer. Clin. Nucl. Med. 2018;43:225–231. doi: 10.1097/RLU.0000000000001991. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

