Boron Chemicals in Drug Discovery and Development: Synthesis and Medicinal Perspective
- PMID: 35565972
- PMCID: PMC9104566
- DOI: 10.3390/molecules27092615
Boron Chemicals in Drug Discovery and Development: Synthesis and Medicinal Perspective
Abstract
A standard goal of medicinal chemists has been to discover efficient and potent drug candidates with specific enzyme-inhibitor abilities. In this regard, boron-based bioactive compounds have provided amphiphilic properties to facilitate interaction with protein targets. Indeed, the spectrum of boron-based entities as drug candidates against many diseases has grown tremendously since the first clinically tested boron-based drug, Velcade. In this review, we collectively represent the current boron-containing drug candidates, boron-containing retinoids, benzoxaboroles, aminoboronic acid, carboranes, and BODIPY, for the treatment of different human diseases.In addition, we also describe the synthesis, key structure-activity relationship, and associated biological activities, such as antimicrobial, antituberculosis, antitumor, antiparasitic, antiprotozoal, anti-inflammatory, antifolate, antidepressant, antiallergic, anesthetic, and anti-Alzheimer's agents, as well as proteasome and lipogenic inhibitors. This compilation could be very useful in the exploration of novel boron-derived compounds against different diseases, with promising efficacy and lesser side effects.
Keywords: ALS; aminoboronic acids; bezoxaboroles; boron-containing drugs; boron-containing retinoids; brain cancer; lipogenic inhibitors; proteasome inhibitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

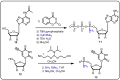
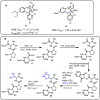

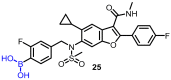
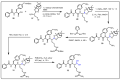




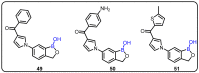
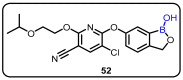






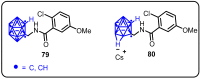


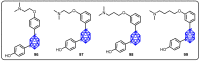





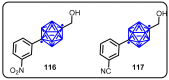






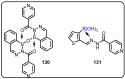

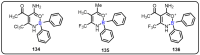
References
-
- Das B.C., Ojha D.P., Das S., Evans T. Boron-Based Compounds: Potential and Emerging Applications in Medicine. John Wiley & Sons; New York, NY, USA: 2018. Boron Compounds in Molecular Imaging; pp. 205–231.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

