Quality Assessment of Artemisia rupestris L. Using Quantitative Analysis of Multi-Components by Single Marker and Fingerprint Analysis
- PMID: 35565985
- PMCID: PMC9099709
- DOI: 10.3390/molecules27092634
Quality Assessment of Artemisia rupestris L. Using Quantitative Analysis of Multi-Components by Single Marker and Fingerprint Analysis
Abstract
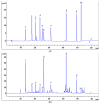
The chromatographic fingerprint of 14 batches of Artemisia rupestris L. samples were established in this study. The constituents of ten components in Artemisia rupestris L. were determined using quantitative analysis of multi-components by single marker (QAMS) and the external standard method (ESM). Due to their stability and accessibility, chlorogenic acid and linarin were used as references to calculate the relative correction factors (RCFs) of apigenin-C-6,8-pentoside-hexoside, apigenin-C-6,8-di-pentoside, luteolin, 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, 4,5-dicaffeoylquinic acid, chrysosplenetin B, and sbsinthin, based on high-performance liquid chromatography (HPLC). The value calculated by QAMS was consistent with that of the ESM, and the reproducibility of RCFs was found to be reliable. In conclusion, simultaneous determination of the ten components by the QAMS method and chromatographic fingerprint analysis were feasible and accurate in evaluating the quality of Artemisia rupestris L. and can be used as reference in traditional Chinese medicine quality control.
Keywords: Artemisia rupestris L.; fingerprint analysis; high-performance liquid chromatography; quality control; quantitative analysis of multi-components by single marker.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Liu Y.M., Liu W.X., Yikemu Y.W.T. Pharmacography of Uighur. Xinjiang People’s Publishing House; Urumqi, China: 1985. pp. 1–5.
-
- Nayak M.K., Daglish G.J., Byrne V.S. Effectiveness of Spinosad as a grain protectant against resistant beetle and psocid pests of stored grain in Australia. J. Stored Prod. Res. 2005;41:455–467. doi: 10.1016/j.jspr.2004.07.002. - DOI
-
- Liu Y.M., Yu D.Q. Studies on chemical constituents in herb from Artemisia rupestris. Acta Pharm. Sin. 1985;20:514–518.
-
- Xu G.S., Chen X.Y., Yu D.Q. Rupestonic acid, a new sesquiterpene from Artemisia rupestris L. Acta Pharm. Sin. 1988;23:122–125.
-
- Song W.X., Ji T.F., Si Y.K., Su Y.L. Studies on chemical constituents in herb from Artemisia rupestris. Chin. J. Chin. Mater. Med. 2006;31:1790–1792.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

