Synthesis and Applications of Nitrogen-Containing Heterocycles as Antiviral Agents
- PMID: 35566055
- PMCID: PMC9101374
- DOI: 10.3390/molecules27092700
Synthesis and Applications of Nitrogen-Containing Heterocycles as Antiviral Agents
Abstract
Viruses have been a long-term source of infectious diseases that can lead to large-scale infections and massive deaths. Especially with the recent highly contagious coronavirus (COVID-19), antiviral drugs were developed nonstop to deal with the emergence of new viruses and subject to drug resistance. Nitrogen-containing heterocycles have compatible structures and properties with exceptional biological activity for the drug design of antiviral agents. They provided a broad spectrum of interference against viral infection at various stages, from blocking early viral entry to disrupting the viral genome replication process by targeting different enzymes and proteins of viruses. This review focused on the synthesis and application of antiviral agents derived from various nitrogen-containing heterocycles, such as indole, pyrrole, pyrimidine, pyrazole, and quinoline, within the last ten years. The synthesized scaffolds target HIV, HCV/HBV, VZV/HSV, SARS-CoV, COVID-19, and influenza viruses.
Keywords: COVID-19; antiviral agents; inhibition; nitrogen-containing heterocycles; synthesis; viruses.
Conflict of interest statement
The authors have no conflict of interest.
Figures



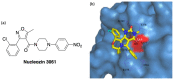

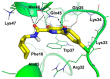
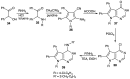
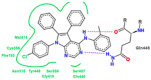

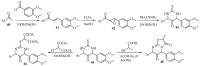
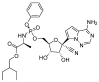





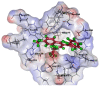
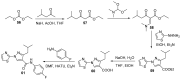

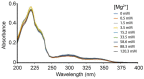


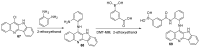

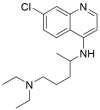

References
-
- Korkmaz A., Bursal E. An in vitro and in silico study on the synthesis and characterization of novel bis(sulfonate) derivatives as tyrosinase and pancreatic lipase inhibitors. J. Mol. Struct. 2022;1259:132734. doi: 10.1016/j.molstruc.2022.132734. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

