Drug Repurposing for COVID-19: A Review and a Novel Strategy to Identify New Targets and Potential Drug Candidates
- PMID: 35566073
- PMCID: PMC9099573
- DOI: 10.3390/molecules27092723
Drug Repurposing for COVID-19: A Review and a Novel Strategy to Identify New Targets and Potential Drug Candidates
Abstract
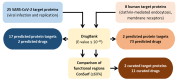
In December 2019, the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19) was first identified in the province of Wuhan, China. Since then, there have been over 400 million confirmed cases and 5.8 million deaths by COVID-19 reported worldwide. The urgent need for therapies against SARS-CoV-2 led researchers to use drug repurposing approaches. This strategy allows the reduction in risks, time, and costs associated with drug development. In many cases, a repurposed drug can enter directly to preclinical testing and clinical trials, thus accelerating the whole drug discovery process. In this work, we will give a general overview of the main developments in COVID-19 treatment, focusing on the contribution of the drug repurposing paradigm to find effective drugs against this disease. Finally, we will present our findings using a new drug repurposing strategy that identified 11 compounds that may be potentially effective against COVID-19. To our knowledge, seven of these drugs have never been tested against SARS-CoV-2 and are potential candidates for in vitro and in vivo studies to evaluate their effectiveness in COVID-19 treatment.
Keywords: COVID-19; SARS-CoV-2; computer-aided drug discovery; drug repurposing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Progress, pitfalls, and path forward of drug repurposing for COVID-19 treatment.Ther Adv Respir Dis. 2022 Jan-Dec;16:17534666221132736. doi: 10.1177/17534666221132736. Ther Adv Respir Dis. 2022. PMID: 36282077 Free PMC article. Review.
-
COVID-19: Potential Repurposing Drugs.Infect Disord Drug Targets. 2022;22(1):e110122191924. doi: 10.2174/1871526521666210301143441. Infect Disord Drug Targets. 2022. PMID: 33645490 Review.
-
Efficacy of repurposed antiviral drugs: Lessons from COVID-19.Drug Discov Today. 2022 Jul;27(7):1954-1960. doi: 10.1016/j.drudis.2022.02.012. Epub 2022 Feb 19. Drug Discov Today. 2022. PMID: 35192924 Free PMC article. Review.
-
Drug repurposing approach to combating coronavirus: Potential drugs and drug targets.Med Res Rev. 2021 May;41(3):1375-1426. doi: 10.1002/med.21763. Epub 2020 Dec 5. Med Res Rev. 2021. PMID: 33277927 Free PMC article. Review.
-
Drugs repurposing for SARS-CoV-2: new insight of COVID-19 druggability.Expert Rev Anti Infect Ther. 2022 Sep;20(9):1187-1204. doi: 10.1080/14787210.2022.2082944. Epub 2022 Jun 6. Expert Rev Anti Infect Ther. 2022. PMID: 35615888
Cited by
-
Repurposed Drugs during the Outbreak of Pandemic COVID-19: A Mini-Review on Their Molecular Structures and Hit-and-Trial Results.ACS Omega. 2024 Aug 20;9(35):36858-36864. doi: 10.1021/acsomega.4c05357. eCollection 2024 Sep 3. ACS Omega. 2024. PMID: 39246499 Free PMC article. Review.
-
DrugProtAI: A machine learning-driven approach for predicting protein druggability through feature engineering and robust partition-based ensemble methods.Brief Bioinform. 2025 Jul 2;26(4):bbaf330. doi: 10.1093/bib/bbaf330. Brief Bioinform. 2025. PMID: 40627683 Free PMC article.
-
Characterization of the symmetrical benzimidazole twin drug TL1228: the role as viral entry inhibitor for fighting COVID-19.Biol Direct. 2024 Oct 16;19(1):93. doi: 10.1186/s13062-024-00523-9. Biol Direct. 2024. PMID: 39415197 Free PMC article.
-
DTSEA: A network-based drug target set enrichment analysis method for drug repurposing against COVID-19.Comput Biol Med. 2023 Jun;159:106969. doi: 10.1016/j.compbiomed.2023.106969. Epub 2023 Apr 21. Comput Biol Med. 2023. PMID: 37105108 Free PMC article.
-
Evaluation of Drug and Herbal Medicinal Promotions on Social Media During the COVID-19 Pandemic in Relation to World Health Organization Ethical Criteria and South African Health Products Regulatory Authority Guidelines in South Africa: Cross-Sectional Content Analysis.Online J Public Health Inform. 2024 Sep 18;16:e58378. doi: 10.2196/58378. Online J Public Health Inform. 2024. PMID: 39293046 Free PMC article.
References
-
- World Health Organization Coronavirus Disease (COVID-19) [(accessed on 11 February 2022)]. Available online: https://www.who.int/news-room/q-a-detail/coronavirus-disease-COVID-19.
-
- WHO Coronavirus (COVID-19) Dashboard. [(accessed on 11 February 2022)]. Available online: https://COVID19.who.int/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous