Novel Chitosan-Based Schiff Base Compounds: Chemical Characterization and Antimicrobial Activity
- PMID: 35566088
- PMCID: PMC9102824
- DOI: 10.3390/molecules27092740
Novel Chitosan-Based Schiff Base Compounds: Chemical Characterization and Antimicrobial Activity
Abstract
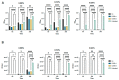
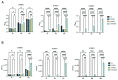
Chitosan (CS) and its derivatives are receiving considerable attention for their great biocompatibility and broad-spectrum activities in many fields. In this work, we aimed to characterize the antimicrobial activity of novel chitosan Schiff bases (CSSB). CS was synthesized by double deacetylation of chitin (Cn) after its extraction from the armors of crustaceans Astacus leptodactylus, and CSSB-1 and CSSB-2 were synthesized by interaction of CS with 4-(2-chloroethyl) benzaldehyde (aldehyde-1) and 4-(bromoethyl) benzaldehyde (aldehyde-2), respectively, at room temperature. The synthesized compounds were characterized by elemental analysis, gel permeation chromatography (GPC), infrared spectroscopy (FTIR), thermogravimetry (TG), and differential scanning calorimetry (DSC). The antimicrobial activity against Gram-positive (Staphylococcus aureus) and Gram-negative (Pseudomonas aeruginosa) bacteria and against yeasts (Candida albicans) was significantly increased due to their higher solubility as compared to unmodified CS opening perspectives for the use of these compounds for antimicrobial prevention in different fields as, for example, food industry, cosmetics, or restoration.
Keywords: antibacterial activity; antifungal activity; chitosan; chitosan-based Schiff base.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Allan C.R., Hadwiger L.A. The fungicidal effect of chitosan on fungi of varying cell wall composition. Exp. Mycol. 1979;3:285–287. doi: 10.1016/S0147-5975(79)80054-7. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

