Comparison of the Phytochemical Variation of Non-Volatile Metabolites within Mother Tinctures of Arnica montana Prepared from Fresh and Dried Whole Plant Using UHPLC-HRMS Fingerprinting and Chemometric Analysis
- PMID: 35566089
- PMCID: PMC9103735
- DOI: 10.3390/molecules27092737
Comparison of the Phytochemical Variation of Non-Volatile Metabolites within Mother Tinctures of Arnica montana Prepared from Fresh and Dried Whole Plant Using UHPLC-HRMS Fingerprinting and Chemometric Analysis
Abstract
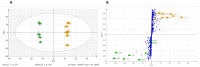
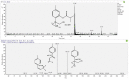
Arnica montana L. has been recognized for centuries as an herbal remedy to treat wounds and promote healing. It also has a long tradition of use in homeopathy. Depending on its medicinal utilization, standardization regulations allow different manufacturing processes, implying different raw materials, such as the whole arnica plant in its fresh or dried state. In this study, an untargeted metabolomics approach with UHPLC-HRMS/MS was used to cross-compare the phytochemical composition of mother tinctures of A. montana that were prepared from either fresh whole plant (fMT) matter or from oven-dried whole plant (dMT) matter. The multivariate data analysis showed significant differences between fMT and dMT. The dereplication of the HRMS and MS/MS spectra of the more discriminant compounds led to annotated quinic acid, dicaffeoyl quinic acids, ethyl caffeate, thymol derivatives and dehydrophytosphingosine, which were increased in fMT, while Amadori rearrangement products (ARP) and methoxyoxaloyl-dicaffeoyl quinic acid esters were enhanced in dMT. Neither sesquiterpene lactones nor flavonoids were affected by the drying process. This is the first time that a sphingosine, ethyl caffeate and ARP are described in A. montana. Moreover, putative new natural products were detected as 10-hydroxy-8,9-epoxy-thymolisobutyrate and an oxidized proline fructose conjugate, for which isolation and full structure elucidation will be necessary to verify this finding.
Keywords: Arnica montana; dried vs. fresh materials; metabolomics; mother tinctures; phytochemical differences.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Tutin T.G., Heywood V.H., Burges N.A., Moore D.M., Valentine D.H., Walters S.M., Webb D.A. Flora Europaea. Plantaginaceae to Compositae (and Rubiaceae) Volume 4. Cambridge University Press; Cambridge, UK: 1976.
-
- ESCOP Monographs. The Scientific Foundation for Herbal Medicinal Products. Second Edition Completely Revised and Expanded. Arnica Flos Monograph. ESCOP and Georg Thieme Verlag; Stuttgart, Germany: 2003. ESCOP 2003.
-
- European Medicines Agency (EMA) Community Herbal Monograph on Arnica Montana. L. Flos. 2014. [(accessed on 10 April 2022)]. Available online: Https://www.Ema.Europa.Eu/En/Documents/Herbal-Monograph/Final-Community-....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

