Design, Synthesis, and Antifungal Activity of 4-Amino Coumarin Based Derivatives
- PMID: 35566096
- PMCID: PMC9104767
- DOI: 10.3390/molecules27092738
Design, Synthesis, and Antifungal Activity of 4-Amino Coumarin Based Derivatives
Abstract
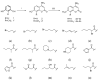
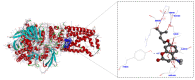
A series of 30 succinate dehydrogenase inhibitors (SDHIs) of 4-amino coumarin-based derivatives were designed and synthesized. According to the analysis of fungicidal activity in vitro, most of the compounds expressed broad-spectrum antifungal activity against four plant pathogenic fungi (Alternaria alternata, Alternaria solani, Fusarium oxysporum, and Botrytis cinerea) using the mycelium growth inhibition method. The results showed that compounds 3n with the group of 2-ene-3-methyl-butyl and 4e with the group of 2-bromo-1-oxo-hexyl displayed excellent activity against Alternaria alternata and Alternaria solani, with EC50 values of 92~145 μg/mL. Molecular docking showed that the inhibitor 3n was completely locked into the cavity of SDH, forming a conventional hydrogen bond interacting with the amino acid residue TYR58. The present work indicates that these derivatives would serve as novel potential fungicides targeting SDH.
Keywords: 4-amino coumarin; antifungal activity; fungicide; inhibitor; molecular docking; succinate dehydrogenase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Mckay A.H., Hagerty G.C., Follas G.B., Moore M.S., Christie M.S., Beresford R.M. Succinate dehydrogenase inhibitor (SDHI) fungicide resistance prevention strategy. N. Z. Plant Prot. 2011;64:119–124. doi: 10.30843/nzpp.2011.64.5972. - DOI
-
- Fellman J.K., Tourneau D.L., Stiers D.L. Effects of some carboxamide fungicides on growth and sclerotium formation of Sclerotium rolfsii. Trans. Br. Mycol. Soc. 1983;80:170–172. doi: 10.1016/S0007-1536(83)80183-1. - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

