Silk Fibroin-Based Biomaterials for Tissue Engineering Applications
- PMID: 35566110
- PMCID: PMC9103528
- DOI: 10.3390/molecules27092757
Silk Fibroin-Based Biomaterials for Tissue Engineering Applications
Abstract
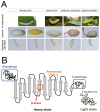
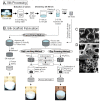
Tissue engineering (TE) involves the combination of cells with scaffolding materials and appropriate growth factors in order to regenerate or replace damaged and degenerated tissues and organs. The scaffold materials serve as templates for tissue formation and play a vital role in TE. Among scaffold materials, silk fibroin (SF), a naturally occurring protein, has attracted great attention in TE applications due to its excellent mechanical properties, biodegradability, biocompatibility, and bio-absorbability. SF is usually dissolved in an aqueous solution and can be easily reconstituted into different forms, including films, mats, hydrogels, and sponges, through various fabrication techniques, including spin coating, electrospinning, freeze drying, and supercritical CO2-assisted drying. Furthermore, to facilitate the fabrication of more complex SF-based scaffolds, high-precision techniques such as micro-patterning and bio-printing have been explored in recent years. These processes contribute to the diversity of surface area, mean pore size, porosity, and mechanical properties of different silk fibroin scaffolds and can be used in various TE applications to provide appropriate morphological and mechanical properties. This review introduces the physicochemical and mechanical properties of SF and looks into a range of SF-based scaffolds that have recently been developed. The typical applications of SF-based scaffolds for TE of bone, cartilage, teeth and mandible tissue, cartilage, skeletal muscle, and vascular tissue are highlighted and discussed followed by a discussion of issues to be addressed in future studies.
Keywords: biomaterial; silk fibroin; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Silk Fibroin as a Functional Biomaterial for Tissue Engineering.Int J Mol Sci. 2021 Feb 2;22(3):1499. doi: 10.3390/ijms22031499. Int J Mol Sci. 2021. PMID: 33540895 Free PMC article. Review.
-
Silk Hydrogel for Tissue Engineering: A Review.J Contemp Dent Pract. 2022 Apr 1;23(4):467-477. J Contemp Dent Pract. 2022. PMID: 35945843 Review.
-
Combinatory approach for developing silk fibroin scaffolds for cartilage regeneration.Acta Biomater. 2018 May;72:167-181. doi: 10.1016/j.actbio.2018.03.047. Epub 2018 Apr 5. Acta Biomater. 2018. PMID: 29626700
-
Silk fibroin aerogels: potential scaffolds for tissue engineering applications.Biomed Mater. 2015 May 8;10(3):035002. doi: 10.1088/1748-6041/10/3/035002. Biomed Mater. 2015. PMID: 25953953
-
Silk fibroin for skin injury repair: Where do things stand?Adv Drug Deliv Rev. 2020 Jan 1;153:28-53. doi: 10.1016/j.addr.2019.09.003. Epub 2019 Oct 31. Adv Drug Deliv Rev. 2020. PMID: 31678360 Review.
Cited by
-
Advancements in skeletal muscle tissue engineering: strategies for repair and regeneration of skeletal muscle beyond self-repair.Regen Biomater. 2025 May 28;12:rbaf050. doi: 10.1093/rb/rbaf050. eCollection 2025. Regen Biomater. 2025. PMID: 40599408 Free PMC article. Review.
-
Material matters: exploring the interplay between natural biomaterials and host immune system.Front Immunol. 2023 Oct 23;14:1269960. doi: 10.3389/fimmu.2023.1269960. eCollection 2023. Front Immunol. 2023. PMID: 37936689 Free PMC article. Review.
-
Hydrogel-Based Scaffolds: Advancing Bone Regeneration Through Tissue Engineering.Gels. 2025 Feb 27;11(3):175. doi: 10.3390/gels11030175. Gels. 2025. PMID: 40136878 Free PMC article. Review.
-
Electrically conductive biopolymer-based hydrogels and fibrous materials fabricated using 3D printing and electrospinning for cardiac tissue engineering.Bioact Mater. 2025 Jun 9;51:650-719. doi: 10.1016/j.bioactmat.2025.05.014. eCollection 2025 Sep. Bioact Mater. 2025. PMID: 40546729 Free PMC article. Review.
-
Rheology as a Tool for Fine-Tuning the Properties of Printable Bioinspired Gels.Molecules. 2023 Mar 19;28(6):2766. doi: 10.3390/molecules28062766. Molecules. 2023. PMID: 36985738 Free PMC article. Review.
References
-
- Kundu B., Kurland N.E., Bano S., Patra C., Engel F.B., Yadavalli V.K., Kundu S.C. Silk proteins for biomedical applications: Bioengineering perspectives. Prog. Polym. Sci. 2014;39:251–267. doi: 10.1016/j.progpolymsci.2013.09.002. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

