Computational Insight into Biotransformation Profiles of Organophosphorus Flame Retardants to Their Diester Metabolites by Cytochrome P450
- PMID: 35566150
- PMCID: PMC9102461
- DOI: 10.3390/molecules27092799
Computational Insight into Biotransformation Profiles of Organophosphorus Flame Retardants to Their Diester Metabolites by Cytochrome P450
Abstract
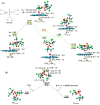
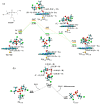
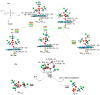
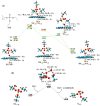
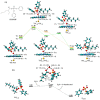
Biotransformation of organophosphorus flame retardants (OPFRs) mediated by cytochrome P450 enzymes (CYPs) has a potential correlation with their toxicological effects on humans. In this work, we employed five typical OPFRs including tris(1,3-dichloro-2-propyl) phosphate (TDCIPP), tris(1-chloro-2-propyl) phosphate (TCIPP), tri(2-chloroethyl) phosphate (TCEP), triethyl phosphate (TEP), and 2-ethylhexyl diphenyl phosphate (EHDPHP), and performed density functional theory (DFT) calculations to clarify the CYP-catalyzed biotransformation of five OPFRs to their diester metabolites. The DFT results show that the reaction mechanism consists of Cα-hydroxylation and O-dealkylation steps, and the biotransformation activities of five OPFRs may follow the order of TCEP ≈ TEP ≈ EHDPHP > TCIPP > TDCIPP. We further performed molecular dynamics (MD) simulations to unravel the binding interactions of five OPFRs in the CYP3A4 isoform. Binding mode analyses demonstrate that CYP3A4-mediated metabolism of TDCIPP, TCIPP, TCEP, and TEP can produce the diester metabolites, while EHDPHP metabolism may generate para-hydroxyEHDPHP as the primary metabolite. Moreover, the EHDPHP and TDCIPP have higher binding potential to CYP3A4 than TCIPP, TCEP, and TEP. This work reports the biotransformation profiles and binding features of five OPFRs in CYP, which can provide meaningful clues for the further studies of the metabolic fates of OPFRs and toxicological effects associated with the relevant metabolites.
Keywords: P450 enzyme; biotransformation; density functional theory calculations; molecular dynamics simulations; organophosphorus flame retardant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Molecular Mechanism of Indoor Exposure to Airborne Halogenated Flame Retardants TCIPP (Tris(1,3-Dichloro-2-Propyl) Phosphate) and TCEP Tris(2-chloroethyl) Phosphate and Their Hazardous Effects on Biological Systems.Metabolites. 2024 Dec 10;14(12):697. doi: 10.3390/metabo14120697. Metabolites. 2024. PMID: 39728479 Free PMC article. Review.
-
Development of a rat physiologically based kinetic model (PBK) for three organophosphate flame retardants (TDCIPP, TCIPP, TCEP).Toxicol Lett. 2023 Jul 1;383:128-140. doi: 10.1016/j.toxlet.2023.06.006. Epub 2023 Jun 24. Toxicol Lett. 2023. PMID: 37356742
-
Prenatal exposure to emerging and traditional organophosphate flame retardants: Regional comparison, transplacental transfer, and birth outcomes.Environ Pollut. 2023 Nov 1;336:122463. doi: 10.1016/j.envpol.2023.122463. Epub 2023 Sep 3. Environ Pollut. 2023. PMID: 37669697
-
Occurrence, Removal, and Environmental Emission of Organophosphate Flame Retardants/Plasticizers in a Wastewater Treatment Plant in New York State.Environ Sci Technol. 2017 Jul 18;51(14):7872-7880. doi: 10.1021/acs.est.7b02035. Epub 2017 Jun 28. Environ Sci Technol. 2017. PMID: 28605181
-
Human internal exposure to organophosphate esters: A short review of urinary monitoring on the basis of biological metabolism research.J Hazard Mater. 2021 Sep 15;418:126279. doi: 10.1016/j.jhazmat.2021.126279. Epub 2021 Jun 5. J Hazard Mater. 2021. PMID: 34329041 Review.
Cited by
-
Biomonitoring urinary organophosphorus flame retardant metabolites by liquid-liquid extraction and ultra-high performance liquid chromatography-tandem mass spectrometry and their association with oxidative stress.Anal Bioanal Chem. 2024 Aug;416(20):4543-4554. doi: 10.1007/s00216-024-05393-8. Epub 2024 Jun 14. Anal Bioanal Chem. 2024. PMID: 38877147
-
Molecular Mechanism of Indoor Exposure to Airborne Halogenated Flame Retardants TCIPP (Tris(1,3-Dichloro-2-Propyl) Phosphate) and TCEP Tris(2-chloroethyl) Phosphate and Their Hazardous Effects on Biological Systems.Metabolites. 2024 Dec 10;14(12):697. doi: 10.3390/metabo14120697. Metabolites. 2024. PMID: 39728479 Free PMC article. Review.
-
New findings on the antagonism of the environmental chemical toxicity 2-ethylhexyl diphenyl phosphate: Glycyrrhizic acid as an Nrf2 activator targets Nrf2/ROS/STAT3 signalling crosstalk to alleviate thymic injury in chicks.Poult Sci. 2025 Apr;104(4):104918. doi: 10.1016/j.psj.2025.104918. Epub 2025 Feb 25. Poult Sci. 2025. PMID: 40024011 Free PMC article.
References
-
- Kim J.-W., Isobe T., Chang K.-H., Amano A., Maneja R.H., Zamora P.B., Siringan F.P., Tanabe S. Levels and distribution of organophosphorus flame retardants and plasticizers in fishes from Manila Bay, the Philippines. Environ. Pollut. 2011;159:3653–3659. doi: 10.1016/j.envpol.2011.07.020. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

