Structural Analysis of the Michael-Michael Ring Closure (MIMIRC) Reaction Products
- PMID: 35566162
- PMCID: PMC9104055
- DOI: 10.3390/molecules27092810
Structural Analysis of the Michael-Michael Ring Closure (MIMIRC) Reaction Products
Abstract
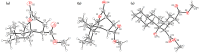
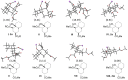
A representative number of decalin and hydrindane derivatives 2a-l were prepared in 11-91% yield by means of a cascade reaction of cyclohexanone/cyclopentanone enolates and methyl acrylate through a Michael-Michael ring closure (MIMIRC) process. The relative stereochemistry of the four stereogenic centers formed in all products was determined by analyzing the vicinal coupling constants from the 1H NMR and X-ray crystallography. Such a stereochemical outcome was corroborated by conformational analysis supported by DFT calculations and simulating the 1H NMR spectra of representative products. All products showed the same relative stereochemistry at C-1 and C-8a, while at C-3 and bridgehead carbon C-4a, configurational changes were observed. The present results provide some insights about the scope and limitations of the triple cascade reaction between cycloalkanone enolates with methyl acrylate. This synthetic protocol is still a simple and very practical alternative to generate decalin and hydrindane derivatives with great structural diversity.
Keywords: cycloalkanone enolates; decalin/hydrindane derivatives; double Michael addition; methyl acrylate; stereochemical outcome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
One-pot organocatalytic enantioselective Michael-Michael-aldol-Henry reaction cascade. A facile entry to the steroid system with six contiguous stereogenic centers.Org Lett. 2014 May 16;16(10):2724-7. doi: 10.1021/ol501011t. Epub 2014 May 5. Org Lett. 2014. PMID: 24796861
-
Diastereo- and enantioselective dearomatization of rhenium-bound naphthalenes.J Org Chem. 2004 Apr 2;69(7):2257-67. doi: 10.1021/jo030318k. J Org Chem. 2004. PMID: 15049617
-
31P NMR parameters may facilitate the stereochemical analysis of phosphorus-containing compounds.J Magn Reson. 2022 Mar;336:107149. doi: 10.1016/j.jmr.2022.107149. Epub 2022 Jan 22. J Magn Reson. 2022. PMID: 35121491
-
[Asymmetric synthesis using chiral bases].Yakugaku Zasshi. 1997 Nov;117(10-11):800-16. doi: 10.1248/yakushi1947.117.10-11_800. Yakugaku Zasshi. 1997. PMID: 9414592 Review. Japanese.
-
New Methods and Strategies in the Synthesis of Terpenoid Natural Products.Acc Chem Res. 2021 Mar 16;54(6):1347-1359. doi: 10.1021/acs.accounts.0c00809. Epub 2021 Feb 17. Acc Chem Res. 2021. PMID: 33596652 Free PMC article. Review.
References
-
- Yuye C., Jing Z., Shaoping L., Jing X. Total synthesis of sesterterpenoids. Nat. Prod. Rep. 2019;36:263–288. - PubMed
-
- Han J., Liu C., Li L., Zhou H., Liu L., Bao L., Chen Q., Song F., Zhang L., Li E., et al. Decalin-Containing Tetramic Acids and 4-Hydroxy-2-pyridones with Antimicrobial and Cytotoxic Activity from the Fungus Coniochaeta cephalothecoides Collected in Tibetan Plateau (Medog) J. Org. Chem. 2017;82:11474–11486. doi: 10.1021/acs.joc.7b02010. - DOI - PubMed
-
- Sharma V., Sharma T., Kaul S., Kapoor K.K., Dhar M.K. Anticancer potential of labdane diterpenoid lactone “andrographolide” and its derivatives: A semi-synthetic approach. Phytochem. Rev. 2017;16:513–526. doi: 10.1007/s11101-016-9478-9. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

