Human Estrogen Receptor Alpha Antagonists, Part 3: 3-D Pharmacophore and 3-D QSAR Guided Brefeldin A Hit-to-Lead Optimization toward New Breast Cancer Suppressants
- PMID: 35566172
- PMCID: PMC9101642
- DOI: 10.3390/molecules27092823
Human Estrogen Receptor Alpha Antagonists, Part 3: 3-D Pharmacophore and 3-D QSAR Guided Brefeldin A Hit-to-Lead Optimization toward New Breast Cancer Suppressants
Abstract
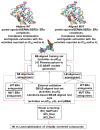
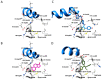
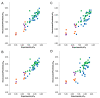
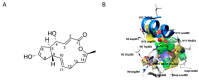
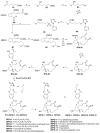
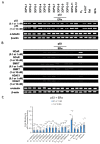
The estrogen receptor α (ERα) is an important biological target mediating 17β-estradiol driven breast cancer (BC) development. Aiming to develop innovative drugs against BC, either wild-type or mutated ligand-ERα complexes were used as source data to build structure-based 3-D pharmacophore and 3-D QSAR models, afterward used as tools for the virtual screening of National Cancer Institute datasets and hit-to-lead optimization. The procedure identified Brefeldin A (BFA) as hit, then structurally optimized toward twelve new derivatives whose anticancer activity was confirmed both in vitro and in vivo. Compounds as SERMs showed picomolar to low nanomolar potencies against ERα and were then investigated as antiproliferative agents against BC cell lines, as stimulators of p53 expression, as well as BC cell cycle arrest agents. Most active leads were finally profiled upon administration to female Wistar rats with pre-induced BC, after which 3DPQ-12, 3DPQ-3, 3DPQ-9, 3DPQ-4, 3DPQ-2, and 3DPQ-1 represent potential candidates for BC therapy.
Keywords: anticancer activity in vitro and in vivo; breast cancer; brefeldin a derivatives synthesis; estrogen receptor α; structure-based 3-D QSAR; structure-based 3-D pharmacophores.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Shiau A.K., Barstad D., Radek J.T., Meyers M., Nettles K.W., Katzenellenbogen B.S., Katzenellenbogen J.A., Agard D.A., Greene G.L. Structural Characterization of a Subtype-Selective Ligand Reveals a Novel Mode of Estrogen Receptor Antagonism. Nat. Genet. 2002;9:359–364. doi: 10.1038/nsb787. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

