Abscisic Acid-Defensive Player in Flax Response to Fusarium culmorum Infection
- PMID: 35566184
- PMCID: PMC9105474
- DOI: 10.3390/molecules27092833
Abscisic Acid-Defensive Player in Flax Response to Fusarium culmorum Infection
Abstract
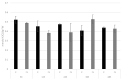
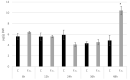
Fusarium culmorum is a ubiquitous soil pathogen with a wide host range. In flax (Linum ussitatissimum), it causes foot and root rot and accumulation of mycotoxins in flax products. Fungal infections lead to huge losses in the flax industry. Moreover, due to mycotoxin accumulation, flax products constitute a potential threat to the consumers. We discovered that the defense against this pathogen in flax is based on early oxidative burst among others. In flax plants infected with F. culmorum, the most affected genes are connected with ROS production and processing, callose synthesis and ABA production. We hypothesize that ABA triggers defense mechanism in flax and is a significant player in a successful response to infection.
Keywords: Fusarium culmorum; abscisic acid; flax; infection; terpenoids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- FAO [(accessed on 1 February 2022)]. Available online: http://www.fao.org/faostat/en/#data/QCL.
-
- Novakovskiy R.O., Dvorianinova E.M., Rozhmina T.A., Kudryavtseva L.P., Gryzunov A.A., Pushkova E.N., Povkhova L.V., Snezhkina A.V., Krasnov G.S., Kudryavtseva A.V., et al. Data on genetic polymorphism of flax (Linum usitatissimum L.) pathogenic fungi of Fusarium, Colletotrichum, Aureobasidium, Septoria, and Melampsora genera. Data Brief. 2020;31:105710. doi: 10.1016/j.dib.2020.105710. - DOI - PMC - PubMed
-
- Boba A., Kostyn K., Kozak B., Wojtasik W., Preisner M., Prescha A., Gola E.M., Lysh D., Dudek B., Szopa J., et al. Fusarium oxysporum infection activates the plastidial branch of the terpenoid biosynthesis pathway in flax, leading to increased ABA synthesis. Planta. 2020;251:50. doi: 10.1007/s00425-020-03339-9. - DOI - PubMed
-
- Pastuszak J., Szczerba A., Dziurka M., Hornyák M., Kopeć P., Szklarczyk M., Płażek A. Physiological and Biochemical Response to Fusarium culmorum Infection in Three Durum Wheat Genotypes at Seedling and Full Anthesis Stage. Int. J. Mol. Sci. 2021;22:7433. doi: 10.3390/ijms22147433. - DOI - PMC - PubMed
-
- Leslie J.F., Summerell B.A., Bullock S. The Fusarium Laboratory Manual. Wiley; Hoboken, NJ, USA: 2006.
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

