Gastric Cancer Pre-Stage Detection and Early Diagnosis of Gastritis Using Serum Protein Signatures
- PMID: 35566209
- PMCID: PMC9099457
- DOI: 10.3390/molecules27092857
Gastric Cancer Pre-Stage Detection and Early Diagnosis of Gastritis Using Serum Protein Signatures
Abstract
Background: A gastric cancer (GC) diagnosis relies on histopathology. Endoscopy rates are increasing. Helicobacter pylori infection is a major GC risk factor. In an effort to elucidate abundant blood biomarkers, and potentially reduce the number of diagnostic surgical interventions, we investigated sera and biopsies from a cohort of 219 H. pylori positive and negative patients diagnosed with GC, gastritis, and ulcers. This allowed the comparative investigation of the different gastroduodenal diseases, and the exclusion of protein changes resulting from bacterial infection or inflammation of the gastric mucosa when searching for GC-dependent proteins.
Methods: High-definition mass spectrometry-based expression analysis of tryptically digested proteins was performed, followed by multivariate statistical and network analyses for the different disease groups, with respect to H. pylori infection status. Significantly regulated proteins differing more than two-fold between groups were shortlisted, and their role in gastritis and GC discussed.
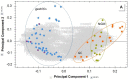
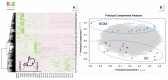
Results: We present data of comparative protein analyses of biopsies and sera from patients suffering from mild to advanced gastritis, ulcers, and early to advanced GC, in conjunction with a wealth of metadata, clinical information, histopathological evaluation, and H. pylori infection status. We used samples from pre-malignant stages to extract prospective serum markers for early-stage GC, and present a 29-protein marker panel containing, amongst others, integrin β-6 and glutathione peroxidase. Furthermore, ten serum markers specific for advanced GC, independent of H. pylori infection, are provided. They include CRP, protein S100A9, and kallistatin. The majority of these proteins were previously discussed in the context of cancer or GC. In addition, we detected hypoalbuminemia and increased fibrinogen serum levels in gastritis.
Conclusion: Two protein panels were suggested for the development of multiplex tests for GC serum diagnostics. For most of the elements contained in these panels, individual commercial tests are available. Thus, we envision the design of multi-protein assays, incorporating several to all of the panel members, in order to gain a level of specificity that cannot be achieved by testing a single protein alone. As their development and validation will take time, gastritis diagnosis based on the fibrinogen to albumin serum ratio may be a quick way forward. Its determination at the primary/secondary care level for early diagnosis could significantly reduce the number of referrals to endoscopy. Preventive measures are in high demand. The protein marker panels presented in this work will contribute to improved GC diagnostics, once they have been transferred from a research result to a practical tool.
Keywords: Helicobacter pylori; gastric cancer; gastritis; proteomics; ulcer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- American Cancer Society. [(accessed on 1 April 2022)]. Available online: www.cancer.org/cancer/stomach-cancer/about/key-statistics.html.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

