New Derivatives of 5-Substituted Uracils: Potential Agents with a Wide Spectrum of Biological Activity
- PMID: 35566215
- PMCID: PMC9102953
- DOI: 10.3390/molecules27092866
New Derivatives of 5-Substituted Uracils: Potential Agents with a Wide Spectrum of Biological Activity
Abstract
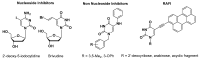
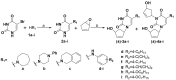
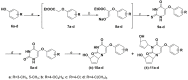
Pyrimidine nucleoside analogues are widely used to treat infections caused by the human immunodeficiency virus (HIV) and DNA viruses from the herpes family. It has been shown that 5-substituted uracil derivatives can inhibit HIV-1, herpes family viruses, mycobacteria and other pathogens through various mechanisms. Among the 5-substituted pyrimidine nucleosides, there are not only the classical nucleoside inhibitors of the herpes family viruses, 2'-deoxy-5-iodocytidine and 5-bromovinyl-2'-deoxyuridine, but also derivatives of 1-(benzyl)-5-(phenylamino)uracil, which proved to be non-nucleoside inhibitors of HIV-1 and EBV. It made this modification of nucleoside analogues very promising in connection with the emergence of new viruses and the crisis of drug resistance when the task of creating effective antiviral agents of new types that act on other targets or exhibit activity by other mechanisms is very urgent. In this paper, we present the design, synthesis and primary screening of the biological activity of new nucleoside analogues, namely, 5'-norcarbocyclic derivatives of substituted 5-arylamino- and 5-aryloxyuracils, against RNA viruses.
Keywords: 5′-norcarbocyclic nucleoside analogues; RNA viruses; chemical synthesis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
5-(1-Substituted) alkyl pyrimidine nucleosides as antiviral (herpes) agents.Curr Med Chem. 2004 Oct;11(20):2749-66. doi: 10.2174/0929867043364388. Curr Med Chem. 2004. PMID: 15544474 Review.
-
Structure-activity relationships of (E)-5-(2-bromovinyl)uracil and related pyrimidine nucleosides as antiviral agents for herpes viruses.J Med Chem. 2000 Jun 29;43(13):2538-46. doi: 10.1021/jm990543n. J Med Chem. 2000. PMID: 10891113
-
Synthesis and biological evaluation of 2',3'-dideoxy-L-pyrimidine nucleosides as potential antiviral agents against human immunodeficiency virus (HIV) and hepatitis B virus (HBV).J Med Chem. 1994 Mar 18;37(6):798-803. doi: 10.1021/jm00032a013. J Med Chem. 1994. PMID: 8145230
-
Synthesis and Antiviral Evaluation of 3'-Fluoro-5'-norcarbocyclic Nucleoside Phosphonates Bearing Uracil and Cytosine as Potential Antiviral Agents.Molecules. 2020 Aug 14;25(16):3708. doi: 10.3390/molecules25163708. Molecules. 2020. PMID: 32823897 Free PMC article.
-
Bicyclic pyrimidine nucleoside analogues (BCNAs) as highly selective and potent inhibitors of varicella-zoster virus replication.J Antimicrob Chemother. 2002 Jul;50(1):5-9. doi: 10.1093/jac/dkf037. J Antimicrob Chemother. 2002. PMID: 12096000 Review.
Cited by
-
Reaction Pattern and Mechanistic Aspects of Iodine and Iodine-Based Reagents in Selenylation of Aliphatic, Aromatic, and (Hetero)Cyclic Systems.Top Curr Chem (Cham). 2024 Apr 8;382(2):12. doi: 10.1007/s41061-024-00459-8. Top Curr Chem (Cham). 2024. PMID: 38589598 Review.
-
New Biocides Based on N4-Alkylcytidines: Effects on Microorganisms and Application for the Protection of Cultural Heritage Objects of Painting.Int J Mol Sci. 2024 Mar 6;25(5):3053. doi: 10.3390/ijms25053053. Int J Mol Sci. 2024. PMID: 38474298 Free PMC article.
-
Synthesis, Herbicidal Activity, Mode of Action, and In Silico Analysis of Novel Pyrido[2,3-d]pyrimidine Compounds.Molecules. 2023 Oct 31;28(21):7363. doi: 10.3390/molecules28217363. Molecules. 2023. PMID: 37959782 Free PMC article.
-
Nucleoside Analogs That Inhibit SARS-CoV-2 Replication by Blocking Interaction of Virus Polymerase with RNA.Int J Mol Sci. 2023 Feb 8;24(4):3361. doi: 10.3390/ijms24043361. Int J Mol Sci. 2023. PMID: 36834771 Free PMC article.
-
The Functional Implications of Broad Spectrum Bioactive Compounds Targeting RNA-Dependent RNA Polymerase (RdRp) in the Context of the COVID-19 Pandemic.Viruses. 2023 Nov 25;15(12):2316. doi: 10.3390/v15122316. Viruses. 2023. PMID: 38140557 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

