Phytochemical Profile of Antibacterial Agents from Red Betel Leaf (Piper crocatum Ruiz and Pav) against Bacteria in Dental Caries
- PMID: 35566225
- PMCID: PMC9101570
- DOI: 10.3390/molecules27092861
Phytochemical Profile of Antibacterial Agents from Red Betel Leaf (Piper crocatum Ruiz and Pav) against Bacteria in Dental Caries
Abstract
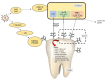
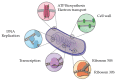
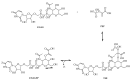
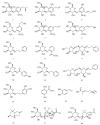
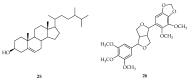
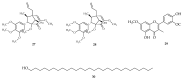
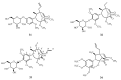
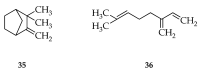
Based on data from The Global Burden of Disease Study in 2016, dental and oral health problems, especially dental caries, are a disease experienced by almost half of the world's population (3.58 billion people). One of the main causes of dental caries is the pathogenesis of Streptococcus mutans. Prevention can be achieved by controlling S. mutans using an antibacterial agent. The most commonly used antibacterial for the treatment of dental caries is chlorhexidine. However, long-term use of chlorhexidine has been reported to cause resistance and some side effects. Therefore, the discovery of a natural antibacterial agent is an urgent need. A natural antibacterial agent that can be used are herbal medicines derived from medicinal plants. Piper crocatum Ruiz and Pav has the potential to be used as a natural antibacterial agent for treating dental and oral health problems. Several studies reported that the leaves of P. crocatum Ruiz and Pav contain secondary metabolites such as essential oils, flavonoids, alkaloids, terpenoids, tannins, and phenolic compounds that are active against S. mutans. This review summarizes some information about P. crocatum Ruiz and Pav, various isolation methods, bioactivity, S. mutans bacteria that cause dental caries, biofilm formation mechanism, antibacterial properties, and the antibacterial mechanism of secondary metabolites in P. crocatum Ruiz and Pav.
Keywords: Piper crocatum Ruiz and Pav; Streptococcus mutans; antibacterial; phytochemical profiling; red betel leaf.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Anti-Infection of Oral Microorganisms from Herbal Medicine of Piper crocatum Ruiz & Pav.Drug Des Devel Ther. 2024 Jun 25;18:2531-2553. doi: 10.2147/DDDT.S453375. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38952486 Free PMC article. Review.
-
Anti-allergic Inflammatory Components from the Leaves of Piper crocatum Ruiz & Pav.Biol Pharm Bull. 2021;44(2):245-250. doi: 10.1248/bpb.b20-00726. Biol Pharm Bull. 2021. PMID: 33518676
-
Isolation of Two New Compounds and Other Constituents from Leaves of Piper crocatum and Study of Their Soluble Epoxide Hydrolase Activities.Molecules. 2019 Jan 30;24(3):489. doi: 10.3390/molecules24030489. Molecules. 2019. PMID: 30704047 Free PMC article.
-
Antibacterial effects of natural compounds on biofilm formation of Streptococcus mutans.Clin Exp Dent Res. 2022 Dec;8(6):1426-1433. doi: 10.1002/cre2.673. Epub 2022 Oct 25. Clin Exp Dent Res. 2022. PMID: 36281582 Free PMC article. Review.
-
Antibacterial Activity of Plant Polyphenols Belonging to the Tannins against Streptococcus mutans-Potential against Dental Caries.Molecules. 2024 Feb 16;29(4):879. doi: 10.3390/molecules29040879. Molecules. 2024. PMID: 38398630 Free PMC article.
Cited by
-
Medicinal Plants Against Dental Caries: Research and Application of Their Antibacterial Properties.Plants (Basel). 2025 May 5;14(9):1390. doi: 10.3390/plants14091390. Plants (Basel). 2025. PMID: 40364419 Free PMC article. Review.
-
Exploring the Inhibitory Potential of M. pendans Compounds Against N-Acetylglucosamine (Mur) Receptor: In Silico Insights Into Antibacterial Activity and Drug-Likeness.ScientificWorldJournal. 2024 Nov 30;2024:3569811. doi: 10.1155/tswj/3569811. eCollection 2024. ScientificWorldJournal. 2024. PMID: 39654692 Free PMC article.
-
Amaranth and quinoa as potential nutraceuticals: A review of anti-nutritional factors, health benefits and their applications in food, medicinal and cosmetic sectors.Food Chem X. 2023 Apr 28;18:100687. doi: 10.1016/j.fochx.2023.100687. eCollection 2023 Jun 30. Food Chem X. 2023. PMID: 37397203 Free PMC article. Review.
-
Antibacterial Activity and Biocompatibility with the Concentration of Ginger Fraction in Biodegradable Gelatin Methacryloyl (GelMA) Hydrogel Coating for Medical Implants.Polymers (Basel). 2022 Dec 5;14(23):5317. doi: 10.3390/polym14235317. Polymers (Basel). 2022. PMID: 36501711 Free PMC article.
-
Anti-Infection of Oral Microorganisms from Herbal Medicine of Piper crocatum Ruiz & Pav.Drug Des Devel Ther. 2024 Jun 25;18:2531-2553. doi: 10.2147/DDDT.S453375. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38952486 Free PMC article. Review.
References
-
- Irani S. Oral Health and Related Factors: An Update. J. Int. Oral Health. 2016;8:1140–1144. doi: 10.2047/Jioh-08-12-19. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

